Aetiology
Most interatrial communications occur sporadically with no family history of congenital heart defects. Familial traits have been reported, caused by mutations in the transcription factors NKX2.5 and TBX5, as well as the structural protein MYH6.[16][17][18] Associations with genetic syndromes (e.g., trisomy 21, Holt-Oram syndrome, Noonan syndrome) are well documented. Girls are more likely than boys to develop interatrial communications. Maternal alcohol consumption increases the risk of developing a range of congenital heart conditions, including interatrial communications.[19]
Pathophysiology
Interatrial blood flow is maintained throughout cardiac embryogenesis while two different septal structures form. The first septum to develop is the septum primum. Programmed cell death in the anterosuperior aspect of the septum primum results in an ostium secundum. The septum secundum then develops to the right of the septum primum as an infolding of the atrial wall forming the limbus of the fossa ovalis.[20] The septum secundum covers the ostium secundum, which results in a valve mechanism allowing only for right-left shunting. The septum primum forms the floor of the fossa ovalis. An abnormality during the development of these structures can result in an interatrial communication.
Secundum atrial septal defects (ASDs) develop due to a mismatch of septum secundum and ostium secundum. Ostium primum defects are endocardial cushion defects preventing adequate fusion with the septum primum. Superior sinus venosus defects have been attributed to the persistence of fetal systemic to pulmonary veno-venous bridges.[21] They are associated with anomalous pulmonary venous drainage of right upper pulmonary veins. Inferior sinus venosus defects can develop between the inferior vena cava and the right atrium.
The direction of blood flow through the defect is related to the relative compliances of the ventricles. In infancy, the right ventricle is relatively thick and non-compliant; therefore, the amount of left-to-right shunt is minimal. However, as the pulmonary vascular resistance decreases, the right ventricle becomes more compliant, increasing the left-to-right shunt. With age, the left-to-right shunt is exacerbated by increasing stiffness of the left ventricle associated with ageing and systemic hypertension.
The right atrium, right ventricle, and pulmonary arteries may enlarge due to the increased volume load. The pulmonary and tricuspid valves may become incompetent due to enlargement of the valves' annuli. Despite the increased volume load, the pulmonary artery pressures are usually only slightly increased. However, the chronic volume overload can cause engorgement of the pulmonary arteries, capillaries, and veins. Some patients develop medial hypertrophy of the pulmonary arteries and pulmonary vascular obstructive disease. Patients become cyanotic if the shunt reverses.
Classification
Classification according to location
Interatrial communications are classified according to their location and the proposed embryogenesis.[2] The true atrial septum comprises the fossa ovalis and a muscular antero-inferior rim. Therefore, only secundum and vestibular ASDs are located in the true atrial septum. Primum defects, sinus venosus defects, and unroofed coronary sinus defects have historically been classed as ASDs due to the left-to-right shunt they produce. However, they are more precisely described as interatrial communications.
Secundum ASDs are found in the region of the fossa ovalis.
Ostium primum defects are found anterior to the fossa ovalis and superior to the atrioventricular valves. These defects are a type of endocardial cushion defect (similar to complete atrioventricular septal defects) and are associated with a cleft in the anterior leaflet of the left atrioventricular valve. Morphologically, this is better described as a 'zone of apposition' of the left atrioventricular valve of the atrioventricular septal defect, and distinctly different from a true cleft in the anterior leaflet of the mitral valve.[3][4]
Sinus venosus defects usually occur superior and posterior to the fossa ovalis, with an intact limbus separating the defect from the fossa. They are associated with anomalous connection of the right pulmonary veins, especially the right superior and middle pulmonary veins. The caval vein (superior or inferior) can override the defect, although the defect may make it difficult to interpret true association to one side or the other.[5][6]
Unroofed coronary sinus defects are found near the orifice of the coronary sinus and are associated with a persistent left superior vena cava draining to the left atrium. Various forms of unroofing have been described.
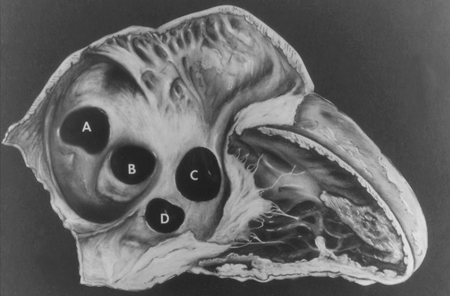
Vestibular defects are found in the muscular antero-inferior rim of the fossa ovalis.[7][Figure caption and citation for the preceding image starts]: Subtypes of interatrial communications. A: sinus venosus defect; B: ostium secundum defect; C: ostium primum defect; D: unroofed coronary sinus defectMayo Clinic Foundation [Citation ends].
Classification by size
The most clinically useful measure of size is the magnitude of the shunt produced by an interatrial communication. This is commonly described as the ratio of pulmonary to systemic blood flow (Qp:Qs ratio). However, symptoms, clinical signs, and evidence of right heart enlargement are frequently driving clinical decisions.
Use of this content is subject to our disclaimer