Approach
The diagnosis of central airway obstruction (CAO) is based on the combination of history (e.g., personal or family history of malignancy), presenting symptoms (common but non-specific, e.g., shortness of breath, cough) and characteristic findings on physical examination, as well as physiological, imaging, and endoscopic studies.[60] Ultimately, the diagnosis is made by direct visualisation of the tracheobronchial obstruction by bronchoscopy, with rigid bronchoscopy being the recommended therapeutic approach.[61]
Airway obstruction presenting with imminent suffocation requires immediate action to promptly and effectively re-establish and secure a patent airway and relieve the obstruction.[18] Rigid bronchoscopy is the procedure of choice in patients with impending respiratory failure, given its capacity for both diagnostic and therapeutic airway intervention.[61] Due to the acuity of the presentation in such patients, investigations that would normally be preliminary (e.g., high-resolution computed tomography, pulmonary function tests) and a diagnostic flexible bronchoscopy may not initially be performed. Thus, in practice, the majority of investigations, particularly pulmonary function tests, are only performed in a minority of patients due to the frequency of emergency presentations.
Clinical history
CAO may present as acutely life-threatening with imminent suffocation or as a progressive respiratory disease. It is thus not possible to take a history in all patients, but, in cases where the presentation allows, a detailed clinical history is essential for diagnosis.
Past medical and surgical history
These must be carefully reviewed and previous medical records obtained.[62]
Any active or previous malignant diseases should be thoroughly researched, as the majority of patients with malignant CAO have an end-stage primary tumour or a recurrence following previous chemo-radiation regimens.[18] A family history of malignancy should also be noted.
A history of critical diseases requiring mechanical ventilation, with or without traumatic intubation or tracheostomy placement, must be investigated.
Previous surgical interventions such as lung resection, lung transplantation, or head and neck surgery must also be investigated.
The presence of rheumatological conditions such as granulomatosis with polyangiitis (formerly known as Wegener's granulomatosis) and relapsing polychondritis are important clues.
Certain infectious diseases such as tuberculosis or histoplasmosis increase the risk of airway stenosis, and acute bacterial tracheitis with involvement of the subglottic area (e.g., diphtheria) may cause airway obstruction due to swelling of mucosa and thick, purulent exudates.
The presence of comorbidities is very important, as patients with CAO often have multiple associated diseases. Active smoking, diabetes, hypertension, and a high score on the American Society of Anesthesiologists (ASA) scale have been found to correlate with treatment-related complications such as intra-operative bleeding and hypoxaemia.[63]
Assessment of aspiration risk
This should be carefully evaluated. Aspiration of foreign bodies leading to CAO is associated with mental status changes, alcohol intoxication, drug overdose, sedative use, seizures, and neurological conditions associated with swallowing impairment such as Parkinson's disease.
Presenting symptoms
Patients with CAO are often quite symptomatic with severely impaired quality of life. The symptomatology of CAO is diverse and non-specific. The most commonly described presenting symptoms are shortness of breath and cough. Other frequently reported symptoms include haemoptysis, hoarseness, chest discomfort, orthopnoea, and dysphagia.[2][3][9][22][34][62][64][65][66]
Shortness of breath is a late sign. It is constant, occurring at rest or on exertion, occasionally positional with laboured breathing in the recumbent position (i.e., orthopnoea) due to structural compression by large intrathoracic tumours, and not responsive to bronchodilators. The degree of shortness of breath does not necessarily correlate with the degree of obstruction, and stenosis may be severe (>70% reduction in cross sectional area) before it causes symptoms at rest or with mild exertion.[1]
Cough is normally chronic, persistent, and dry, but may present acutely in foreign-body aspiration or be productive of purulent sputum in post-obstructive pneumonia.
If the obstruction is mild, patients may be asymptomatic, as the airflow limitation is not significant. However, even in mild obstructions, during an acute respiratory infection, the associated oedema and accumulation of secretions may critically decrease the airway lumen at the stenosis site, leading to rapid respiratory deterioration.[2]
When the airflow limitation reaches a critical point, symptoms develop, and up to 54% of tracheal stenosis patients present in respiratory distress.[2] The diameter of the tracheal lumen must be <8 mm for dyspnoea on exertion to develop, and <5 mm for dyspnoea at rest.[2][62][65] Individuals with pre-existing lung disease (e.g., COPD) may become symptomatic with a lesser degree of obstruction.
Abrupt-onset dyspnoea may indicate foreign-body aspiration. The classical triad of cough, wheezing, and choking in foreign-body aspiration is only present in a small percentage of adults, as most aspirated particles do not completely obstruct the airway and wedge distally (most commonly in the right lower lobe bronchus).[49] However, in children, obstruction is usually in the central airways.[49]
Haemoptysis is common, especially in tracheal lesions, and may be massive, although most studies report mild to moderate haemoptysis. Squamous cell carcinoma, primary airway tumours (carcinoid tumours and adenoid cystic carcinoma), endobronchial metastases, infections (e.g., tuberculosis), inflammatory diseases (e.g., granulomatosis with polyangiitis [formerly known as Wegener's granulomatosis]), and benign airway tumours (e.g., hamartoma) frequently cause haemoptysis. Chronic bronchitis frequently presents with blood-streaked, purulent secretions, which may be misleading in the diagnosis of CAO.[67]
Dysphagia may be present in patients with large airway malignancies causing oesophageal compression, or with oesophageal malignancies with endobronchial invasion.
Tracheal stenosis secondary to endotracheal intubation leads to shortness of breath, cough, and hoarseness within 5 weeks of extubation. Hoarseness is also associated with upper airway malignancies.
Recurrent pneumonias are frequent, presenting with dyspnoea, purulent sputum, fevers, and chills.[66]
A high index of suspicion is required for the diagnosis of granulation tissue-related tracheal obstruction in mechanically ventilated or tracheotomy patients. In these patients, failure to wean, high peak airway pressures on the ventilator, and the development of stridor or increased shortness of breath after artificial airway removal or tracheotomy tube capping are diagnostic clues.
Physical examination
Respiratory examination
On chest auscultation, stridor, wheeze, localised crackles, or frank consolidation may be present, depending on the location, size, and underlying aetiology of the CAO.
Stridor develops when the airway diameter is <5 mm and represents severe subglottic or tracheal stenosis.[68] Inspiratory stridor suggests extrathoracic airway obstruction at or above the vocal cords and is best heard over the neck, while expiratory stridor may be due to an intrathoracic obstruction.[15][62] Biphasic stridor is present in subglottic or tracheal stenosis.[15] Manoeuvres that increase airflow, such as hyperventilation, may accentuate the stridor, and neck flexion may change its intensity.[35]
Wheeze may be inspiratory or expiratory. The location of wheeze does not always conform to the site of the airflow obstruction, and it may be heard over the trachea or lung fields.[68] Unilateral wheeze suggests obstruction distal to the carina. Wheeze may also be positional and is not responsive to bronchodilators.[2][9]
In severe CAO presentations, extreme anxiety, tachypnoea, tachycardia, accessory muscle use, sternal retraction, neck extension, and cyanosis may be seen and may herald an impending respiratory arrest.
Initial imaging
A plain chest x-ray should be obtained in every patient suspected to have CAO.[2][69][70] Although infrequently diagnostic and with a sensitivity to detect abnormalities of the trachea and main bronchi of approximately 66%, chest x-rays may reveal obvious pathologies such as tracheal deviation from an adjacent lesion.[14][71]
The hallmark of airway obstruction on chest x-ray is the finding of air trapping on an exhalation phase film. Air trapping is characterised by failure of the lung to decrease in volume and increase in opacity on exhalation compared with inhalation, as well as mediastinal shift to the side that is not trapping air. Air trapping occurs when an endobronchial lesion causes a check-valve type of obstruction.[71]
Τracheobronchial filling defects may be seen and are indicative of mucus or neoplasms. Another common finding, especially of central squamous cell carcinoma, is post-obstructive pneumonia and atelectasis. In adults with lobar atelectasis, a central obstructive neoplasm should always be considered.[71] In addition, lobar or total lung collapse result from tumour-related obstruction of a main bronchus.
The 'Golden S sign' has been associated with the presence of a central obstructive mass. When a lobe collapses around a large central mass, the peripheral lung collapses and the central portion of the lung is prevented from collapsing by the presence of the mass. The related lung fissure is concave towards the lung peripherally but convex centrally, and the shape of the fissure resembles an 'S' or a 'reverse S'.[71][Figure caption and citation for the preceding image starts]: Golden-S or reverse S-sign on chest CT (left image) traced by yellow line. Flexible bronchoscopy (right image) shows the central obstructive lesion at the left mainstem bronchi.From the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].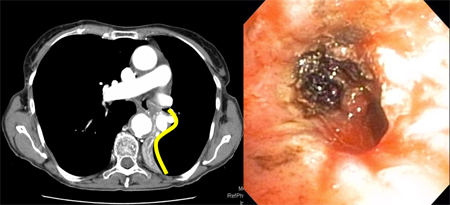
Bronchoscopy
Bronchoscopy (flexible or rigid) is the most specific and sensitive test for diagnosing CAO and is necessary for the assessment of airway obstruction.[9][22][69][72] However, for any therapeutically intended relief of obstruction, rigid bronchoscopy is the preferred approach.[61]
Direct visualisation via bronchoscopy can provide information on the location and morphology of the lesion, the amount of intraluminal disease, the presence of extraluminal compression, and the diameter and length of the lesion. It also allows evaluation of the surrounding tissue, in particular the airway distal to the obstruction. Additionally, tissue can be obtained for pathological diagnosis if necessary.[2]
Diagnostic flexible bronchoscopy has a number of drawbacks and should be performed with caution. It is not able to provide complete evaluation of extraluminal disease or of the airways distal to the obstruction. It may also be difficult and potentially dangerous in severe obstruction, as the bronchoscope itself will further obstruct the already narrow lumen, thus limiting ventilation.[18] The narrow lumen is also at risk of subsequent occlusion following flexible bronchoscopy if secretions, swelling, or bleeding occur at the stricture site. The moderate sedation required during the procedure may decrease ventilation and relax the respiratory muscles, creating a potentially unstable airway. For these reasons, a team equipped for advanced airway management is essential when diagnostic flexible bronchoscopy is performed in CAO.[2]
Bronchoscopic intervention in CAO frequently involves a two-step approach with an initial diagnostic bronchoscopy followed by a therapeutic bronchoscopy, assuming initial airway stability. Flexible bronchoscopy may be used in stable patients for diagnosis, though rigid bronchoscopy is the preferred approach for any therapeutic intervention.[61]
In severe obstruction presenting with impending respiratory failure, diagnostic studies including preliminary flexible bronchoscopy may not be possible, and a rigid bronchoscopy should be performed immediately.[60][69] Rigid bronchoscopy is very useful in the evaluation and management of CAO, as it is a safe and effective way of securing the airway, which provides the ability to ventilate and oxygenate the patient while undertaking diagnostic and therapeutic airway interventions.[16][69][72][73][74][75] In addition, the flexible bronchoscope may be introduced through the rigid scope if required.
Further advanced imaging
Computed tomography (including high-resolution CT, multidetector CT, and virtual bronchoscopy)
CT of the thorax is the most accurate non-invasive method of airway evaluation, allowing diagnosis and treatment planning in CAO.[60][70]
High-resolution CT is considered the imaging test of choice in diagnosing CAO.[69] CT as an imaging modality is superior to chest x-ray in detecting tracheal and main bronchi abnormalities, with a reported sensitivity of 97%.[71]
CT allows determination of the type of obstructive lesion (intraluminal, extrinsic, or mixed) and the patency of the airway distal to the obstruction, the length and diameter of the lesion, and its relationship to nearby structures including vessels.[2]
Tracheal stenosis is defined as focal or diffuse narrowing of the tracheal lumen. Although CT images provide a precise anatomical display of the tracheal wall and lumen, they have limited ability to detect subtle airway stenoses and often underestimate the length of tracheal and bronchial stenoses.
Intratracheal masses and evidence of extrinsic tracheal compression, as well as other benign diffuse tracheal diseases, may be evident.[76] Post-obstructive pneumonia, atelectasis, and lobar collapses may also be visible.
Tracheobronchial filling defects, indicating an intrabronchial space-occupying lesion (e.g., tumour or mucus), may be detected and their location and extent within and around the central airways determined.
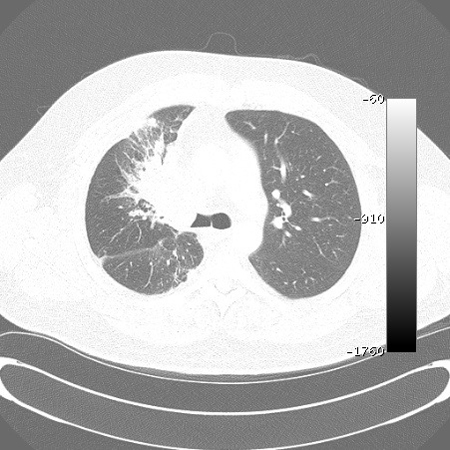
Multidetector CT (MDCT) has the ability to acquire contiguous and/or overlapping high-resolution sections of between 0.5 mm and 2 mm thickness throughout the thorax in a single holding of breath, thus resulting in enhanced detection of airway lesions such as endobronchial tumours (including those in the central airways) that may be missed by conventional CT using 7-10 mm cuts.[41][Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: lung window demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: mediastinal window demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: mediastinal window demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
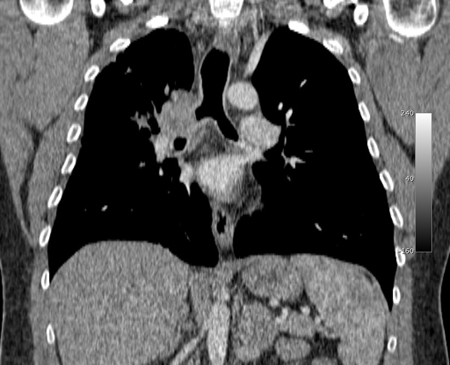
MDCT allows the high-quality reconstruction techniques of multiplanar reconstructions (MPRs), external representation with 3D shaded surface displays, and volumetric representation with internal rendering (the so-called virtual bronchoscopy), which provide more accurate estimations of the length of tracheal and bronchial lesions.[41][77][Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: coronal reconstruction demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: 3D volume rendering reconstruction demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: 3D volume rendering reconstruction demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].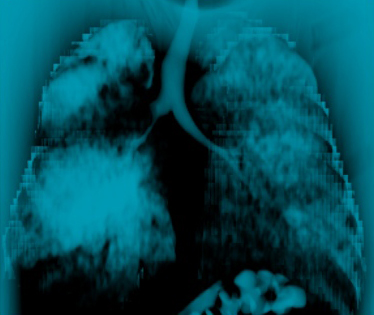 [Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: 2D multiplanar reconstruction (minimal intensity projection) demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Malignant endobronchial obstruction on multidetector chest CT: 2D multiplanar reconstruction (minimal intensity projection) demonstrating right mainstem malignant obstructionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].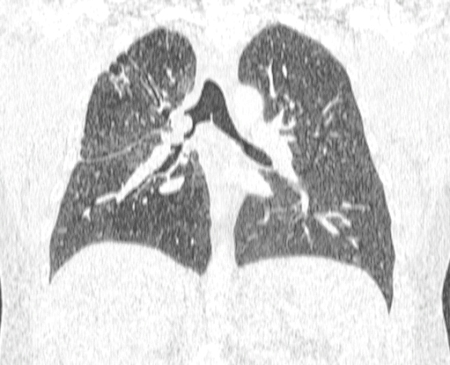
The non-invasive technique of virtual bronchoscopy is comparable to flexible bronchoscopy for the visualisation of the central airways and detection of obstructive bronchial lesions (both endoluminal and external) and focal airway stenoses.[41][78][79] It may also help in the planning of interventional procedures such as airway stenting.[79]
CT and MPRs accurately demonstrate the site and degree of tracheal and main bronchi stenoses with a sensitivity of 93%, specificity of 100%, and accuracy of 94%.[80] The detection accuracy of bronchial stenosis post-lung transplantation is 94%.[81]
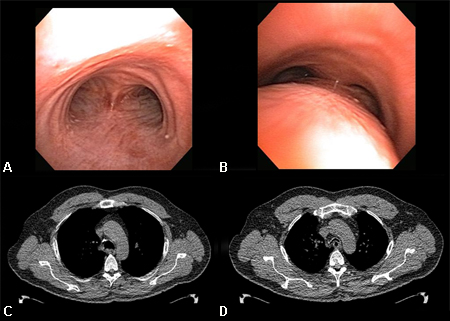
Paired inspiratory dynamic and expiratory multislice helical CT of the chest is useful in the diagnosis of tracheobronchomalacia.[13] Malacia of the central airways is defined as a reduction in the cross-sectional area of >50% on expiratory images compared with inspiratory images.[77][1][Figure caption and citation for the preceding image starts]: Dynamic airway collapse: A. bronchoscopic view on inhalation; B. bronchoscopic view on exhalation showing dynamic airway collapse; C. CT chest showing normal airway on inhalation; D. CT chest showing significant airway collapse on exhalationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
Chest magnetic resonance imaging (MRI)
Chest MRI is useful in assessing the larynx, proximal trachea, and mediastinal and hilar masses, and to readily differentiate between vascular and soft-tissue masses.[69]
It is able to provide multiplane images of the chest without the need of contrast material, and is particularly good at visualising vascular structures surrounding the airways such as vascular rings or aneurysms that may compress the trachea.[35]
Pulmonary function testing
Spirometric and flow-volume loop analysis are an important part of the CAO evaluation and, when possible, these pulmonary function tests should be obtained.
Flow-volume loop (FVL)
Assessment of the shape of the FVL allows identification and categorisation of upper airway obstruction (UAO) as dynamic (non-fixed or variable) extrathoracic, dynamic (non-fixed or variable) intrathoracic, or fixed UAO. The trachea is divided into extrathoracic (one-third of the trachea above the suprasternal notch) and intrathoracic (two-thirds of the trachea below the sternal notch) segments.
In dynamic extrathoracic UAO, during forced inspiration, the extrathoracic portion of the trachea may have an accentuated folding and slower airflow, creating a 'flattened' inspiratory limb.[14] In dynamic intrathoracic UAO, during forced exhalation, the intrathoracic portion of the trachea may have an accentuated folding and slower airflow, creating a 'flattened' expiratory limb.[14] In fixed obstructive lesions of the upper airways, the limitation to airflow does not change in response to transmural pressure changes during inspiration and expiration, creating 'flattened' inspiratory and expiratory loops.[14][Figure caption and citation for the preceding image starts]: Dynamic (non-fixed or variable) extrathoracic upper airway obstruction: flow-volume loop shows 'flattened' inspiratory limb during forced inspirationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
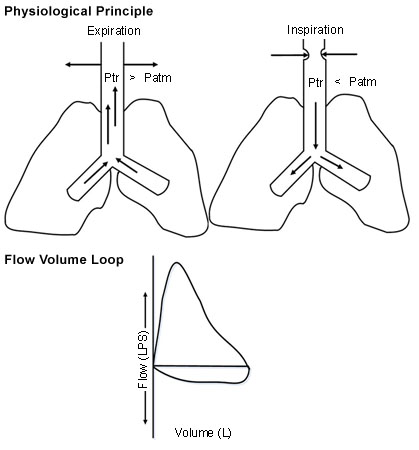 [Figure caption and citation for the preceding image starts]: Dynamic (non-fixed or variable) intrathoracic upper airway obstruction: flow-volume loop shows 'flattened' expiratory limb during forced exhalationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Dynamic (non-fixed or variable) intrathoracic upper airway obstruction: flow-volume loop shows 'flattened' expiratory limb during forced exhalationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].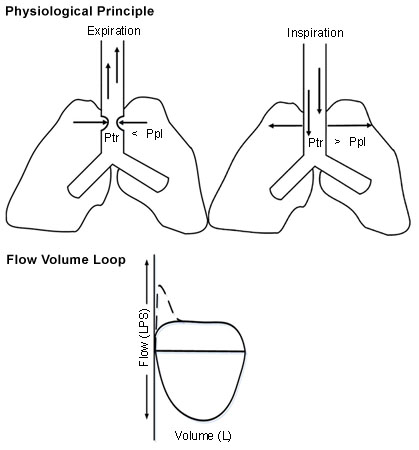 [Figure caption and citation for the preceding image starts]: Fixed upper airway obstruction: flow-volume loop shows 'flattened' inspiratory and expiratory loopsFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Fixed upper airway obstruction: flow-volume loop shows 'flattened' inspiratory and expiratory loopsFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].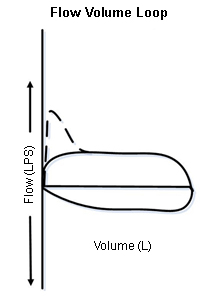
FVL takes into account the dynamic characteristics of the airways, such as alterations in upper airway calibre due to changes in the transmural pressure induced by forced inspiratory and expiratory manoeuvres during spirometry.
As the classical tracings of tracheal obstruction are not seen until the lumen is severely narrowed (diameter 6-10 mm), FVL is not very sensitive in detecting UAO.[1][2][35]
In patients with pre-existing lung diseases (e.g., COPD), FVL may not show the described configuration due to the inability to generate high enough flow rates and the presence of airflow obstruction in multiple anatomical sites.[2] Thus, central lesions are under-recognised with FVL in these patients.[15]
Spirometry
Due to the possibility of inducing respiratory failure, spirometry should not be undertaken in patients with respiratory distress or advanced CAO.[2]
Significant reductions in the forced expiratory volume in 1 second (FEV1) are not observed until the diameter at the obstruction site decreases to 6 mm. Thus, FVL changes may be seen prior to the development of an abnormal FEV1.[2]
Peak expiratory flow rate (PEFR) and maximal voluntary ventilation (MVV) may be more sensitive than FEV1 for the detection of UAO.
If PEFR is reduced disproportionately to the reduction in FEV1 on forced spirometry, UAO should be suspected.[15][35] A ratio of MVV to FEV1 of less than 25% is often seen in UAO, and the diagnosis should be considered when MVV is reduced in association with a normal FEV1.[35]
Endobronchial ultrasound (EBUS)
EBUS has been used successfully in the assessment and treatment planning of CAO.[2][82] Although not routinely used in clinical practice, it can be used to determine the presence of extracartilaginous disease, the degree of airway involvement, and identify the distal end of an obstructive lesion, once the obstruction has been acutely stabilised.[1]
Use of this content is subject to our disclaimer