Approach
A high level of suspicion is warranted if characteristic respiratory signs and symptoms accompany an underlying disorder (e.g., inflammatory bowel disease, connective tissue disorder). Sputum analysis aids diagnosis; however, high-resolution chest computed tomography (CT) is the confirmatory test.
History
Adults
Patients typically present with:
Persistent productive cough
Daily mucopurulent sputum
Dyspnoea[48]
Fatigue
Rhinosinusitis[49]
Blood-tinged sputum (less common)
Haemoptysis (less common).
An acute exacerbation often presents as worsening of cough, change in sputum colour, increase in sputum volume, fever, and/or fatigue. More than half of patients with bronchiectasis will have recurrent episodes of fever, and a history of weight loss is also common.
Children
Mild bronchiectasis is reversible in children if it is diagnosed early.[10] Exacerbations predominantly present with increased cough and increased sputum quantity and/or purulence. Systemic symptoms such as malaise, fatigue, or change in appetite or behaviour may precede an exacerbation but are not specific. Less common symptoms such as dyspnoea, chest pain, haemoptysis, and wheeze may not be present. The presence of dyspnoea and/or hypoxia signify a severe exacerbation, irrespective of their duration.[10]
Physical examination
There may be fever and weight loss. Clubbing of the digits is less common.[48]
Auscultation reveals:
Crackles
High-pitched inspiratory squeaks
Rhonchi
Wheezing.
Pulse oximetry may reveal hypoxaemia in advanced bronchiectasis.
In children and adolescents, changes in chest auscultation are important but should not be relied upon as they are not always present.[10]
Auscultation sounds: Expiratory wheeze
Auscultation sounds: Early inspiratory crackles
Laboratory investigations
Laboratory tests are geared towards identifying the underlying aetiology, as treatment will be successful only if it treats both the bronchiectasis and any underlying disease process. Certain tests are only available in specialised centres.
Full blood count (FBC)
The white blood cell count aids in determining the presence of a superimposed infection or exacerbation.
If the eosinophil count is high, underlying allergic bronchopulmonary aspergillosis is possible.
Sputum culture and sensitivity
Specimens for bacterial, fungal, and acid-fast bacilli cultures are recommended for all patients.
Single or multiple pathogens may be present.
Aids in guiding antibiotic selection.
Frequently, a pathogenic organism can be recovered in the sputum. There may be single or multiple pathogens present. The most common gram-negative organism is Pseudomonas aeruginosa, present in about 25% of patients; it may be in mucoid form. Mucoid P aeruginosa has been shown to correlate with lower pulmonary function and is a marker for severe lung damage.[50][51] It requires special attention because it is more likely to be resistant to antibiotics than non-mucoid Pseudomonas species.[52]
Specimens for bacterial culture may be requested as 'cystic fibrosis bacterial culture' in patients who are at high risk for Pseudomonas or who have had Pseudomonas growth in sputum cultures in the past. This will allow for special vigilance for mucoid-type Pseudomonas, which has been shown to correlate with lower pulmonary function. A cystic fibrosis bacterial culture employs separate agars to select for gram-negative bacterial growth, as well as Burkholderia cepacia. Sensitivities for these cultures are done by manual disc diffusion because mucoid Pseudomonas does not grow well by automated methods.
Gram-positive cocci (Staphylococcus aureus, Streptococcus pneumoniae, or beta-hemolytic streptococci) represent about 18% of positive sputum cultures.
Other common gram-negative organisms are Haemophilus influenzae, Klebsiella pneumonia, Moraxella catarrhalis, and Serratia marcescens. Mycobacteria are commonly isolated as well.
Referral to a specialist familiar with treatment of non-tuberculous mycobacterium (NTM) is recommended if culture is positive for an NTM organism.[48]
Other organisms that may be cultured include fungi (especially Aspergillus) and Nocardia. These may not initially be of clinical significance but may require treatment if they are repeatedly cultured from an individual patient.[53][54]
Sweat sodium chloride concentration and genetic testing for cystic fibrosis transmembrane conductance regulator (CFTR) mutation analysis
Recommended for all children and for adults in whom there is a high index of suspicion for cystic fibrosis because patients may present with variant forms of cystic fibrosis in adulthood.
Minimum testing for a diagnosis of cystic fibrosis to be made should include two measurements of sweat chloride and then CFTR mutation analysis.[55]
If concentration of sodium chloride in patient's sweat is high (≥60 mmol/L [≥60 mEq/L]), a diagnosis of cystic fibrosis is likely.[55]
Factors for a high index of suspicion for cystic fibrosis include:[46]
Early age at onset
Persistent isolation of Staphylococcus aureus on sputum culture
Features of malabsorption
Male primary infertility
Upper lobe bronchiectasis
A history of childhood steatorrhoea.
Serum alpha-1 antitrypsin phenotype and level
Recommended to identify alpha-1 antitrypsin disease as underlying aetiology in patients with co-existing basal panacinar emphysema.[46]
If abnormal result, referral to a pulmonary specialist for consideration of replacement therapy is indicated.
The MM phenotype indicates patient is homozygous for the normal M allele.[56]
Immunoglobulin levels (serum total IgG, IgM, and IgA), IgG subclasses (IgG1, IgG2, IgG3, IgG4), and response to Pneumovax vaccine with Strep pneumo 23 serotype titres[46][57]
Recommended to identify individual immunoglobulin deficiencies.
Immunoglobulin replacement reduces the frequency of infectious episodes and prevents further destruction of the airways.
Specific IgE or skin prick test to Aspergillus fumigatus
Recommended in all patients, in conjunction with FBC and serum total IgE, to investigate for allergic bronchopulmonary aspergillosis.[46]
A diagnosis of allergic bronchopulmonary aspergillosis warrants referral to a pulmonary specialist.
HIV antibody test
Recommended in all patients.
Patients with HIV infection are predisposed to developing recurrent sinopulmonary infections and bronchiectasis, which is likely to be due to abnormal B-lymphocyte function.[58]
Rheumatoid factor
Recommended in all patients.
Bronchiectasis is more common in rheumatoid arthritis population versus general population.
Bronchiectasis symptoms may precede other systemic rheumatoid arthritis findings.[38]
Nasal nitric oxide (NNO) test
Recommended for the diagnosis of primary ciliary dyskinesia (PCD) in cooperative patients 5 years or older with a clinical phenotype consistent with PCD and with cystic fibrosis excluded.[35] It is recommended over transmission electron microscopy and/or genetic testing in this group of patients.[35][59]
Sensitivity of 97% and specificity of 90% for PCD.
A low result (e.g., <100 parts per billion) indicates a diagnosis of PCD, if cystic fibrosis is excluded.[35] A high NNO virtually excludes PCD. This test is not available in all centres.
A low NNO should be followed up with confirmatory testing because other conditions such as cystic fibrosis may present with low NNO.
Additional corroborative testing, such as extended genetic panel testing or microscopy of ciliary structure, may be used to confirm a diagnosis of PCD.[35] Nasal or bronchial tissue is analysed by video microscopy to evaluate ciliary beat motion and, under electron microscopy, to evaluate ciliary ultrastructure.[35] Lipid-laden macrophages seen in bronchial biopsy specimens suggest aspiration. Extended genetic panel testing (>12 genes) can be used as a diagnostic test in patients with a high probability of having PCD.[35]
These tests for PCD are done at specialist centres.
In children and adolescents, the European Respiratory Society guidelines recommend a minimum panel of laboratory tests that should include:[10]
Sweat test
FBC
Immunological tests (total IgG, IgA, IgM, IgE, and specific antibodies to vaccine antigens)
Lower airway bacteriology.
Selected further tests should be based on clinical presentation, including:[10]
Additional in-depth immunological tests (in consultation with a paediatric immunologist)
Diagnostic bronchoscopy with bronchoalveolar lavage (may be used to exclude a foreign body and to obtain microbiological samples)
Tests for aspiration, primary ciliary dyskinesia, and GORD
Tuberculosis testing in high-prevalence settings or with a history of close contact with tuberculosis
HIV testing in high-prevalence settings or where there is clinical suspicion.
Radiographical studies
Chest x-ray is not recommended for diagnosis as chest x-ray findings are non-specific in bronchiectasis and may even be normal. Nevertheless, large lung volumes, areas of consolidation (due to impacted mucus), tram lines, and tubular or ovoid opacities may be present. Thin-walled ring shadows with or without fluid levels may be present as well. [Figure caption and citation for the preceding image starts]: Chest x-ray with dilated and thickened airwaysFrom archives of Dr Sangeeta M. Bhorade; used with permission [Citation ends].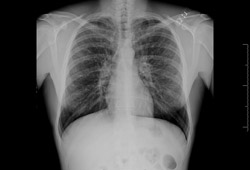 [Figure caption and citation for the preceding image starts]: Chest x-ray with lack of normal tapering producing a tram lineFrom archives of Dr Sangeeta M. Bhorade; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Chest x-ray with lack of normal tapering producing a tram lineFrom archives of Dr Sangeeta M. Bhorade; used with permission [Citation ends].
Although chest CT scan is the diagnostic procedure of choice, a baseline chest x-ray may provide a useful comparator if there is subsequent clinical deterioration.[46] In a child with a chronic wet cough, a chest x-ray can be used to exclude other causes, such as a foreign body or pneumonia.[60]
The US and the UK guidelines recommend thin section CT for the diagnosis of bronchiectasis in adults.[46][61] In children and adolescents, high-resolution multidetector CT (MDCT) scans and a lower diagnostic threshold (broncho-arterial dilatation >0.8 vs. the adult cut-off of >1 to 1.5) are recommended due to the importance of early diagnosis on improving prognosis.[10] Axial CT sections show dilation of bronchi with or without airway thickening. In cross-section, the dilated bronchus will be wider than its accompanying pulmonary artery and resemble a signet ring. Abnormal bronchial dilation is recognised as lack of normal tapering, producing a tram line or flared appearance in the periphery of the lung, and is more prominent when the bronchial walls are thickened. Affected small airways plugged by mucus appear as small, irregular, V- and Y-shaped markings and are about 2 mm in size in the periphery of the lung. This is referred to as a tree-in-bud pattern.[62] CT may show fluid-filled cysts; these represent superimposed infection and warrant a course of systemic antibiotics.[Figure caption and citation for the preceding image starts]: Chest computed tomography scan with dilated and thickened airways and peripheral tree-in-bud patternFrom archives of Dr Sangeeta M. Bhorade; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Chest computed tomography scan with presence of signet ring on leftFrom archives of Dr Sangeeta M. Bhorade; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Chest computed tomography scan with presence of signet ring on leftFrom archives of Dr Sangeeta M. Bhorade; used with permission [Citation ends].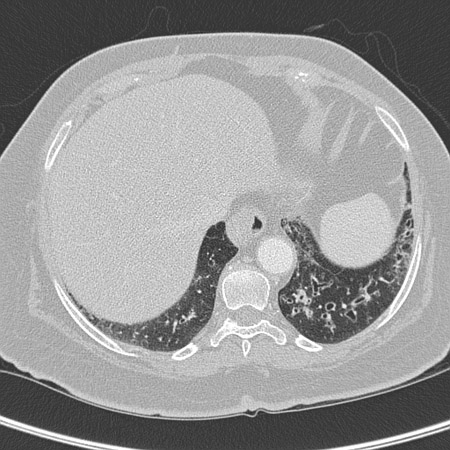 [Figure caption and citation for the preceding image starts]: Signet ring signs in a 20-year-old woman with bronchiectasisFrom Pamela J. McShane, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Signet ring signs in a 20-year-old woman with bronchiectasisFrom Pamela J. McShane, MD; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Severe cystic and varicose bronchiectasis in a 49-year-old man with idiopathic bronchiectasis and scoliosisFrom Pamela J. McShane, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Severe cystic and varicose bronchiectasis in a 49-year-old man with idiopathic bronchiectasis and scoliosisFrom Pamela J. McShane, MD; used with permission [Citation ends].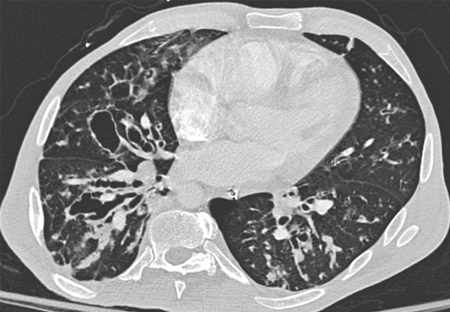
Pulmonary function tests
Spirometry is recommended with most office visits for those patients old enough to co-operate with the test. Obstructive airways disease may be evidenced by reduced forced expiratory volume in the first second of expiration (FEV₁) or an FEV₁/forced vital capacity (FVC) ratio of <70%.[48] Reduction of the FEV₁ suggests the presence of infection or worsening bronchiectasis.
Full pulmonary function testing may show an elevation of residual volume (RV)/total lung capacity (TLC) ratio consistent with air trapping. Diffusing capacity for carbon monoxide (DLCO) may be reduced in severe disease.
Additional work-up
A swallow study or pH monitoring to evaluate for chronic aspiration is also suggested. If these suggest the presence of aspiration, referral to a gastroenterologist and/or ear, nose, and throat specialist is recommended.
A 6-minute walk test should be considered for patients at risk of desaturation with exercise. Can be used to assess serial changes in functional capacity.
Use of this content is subject to our disclaimer

