Etiology
Dupuytren contracture is a progressive fibroproliferative disease that is believed to show autosomal dominant inheritance with variable penetrance.[1] The higher prevalence of the disease in men is probably due to the fact that androgen receptors are expressed in Dupuytren nodules.
The familial occurrence and its presence in identical twins suggest a genetic basis for the disease. The sibling recurrence risk ratio equals 2.9 (95% CI 2.6 to 3.3). Although the genetic factors involved have not been fully elucidated, in one family the gene has been mapped to chromosome 16q.[1] DNA microarray has demonstrated that >30 unique genes are upregulated and 6 unique genes downregulated by a factor of 4 or greater in affected patients.[11] A novel gene, MafB (musculoaponeurotic fibrosarcoma oncogene homolog B), is overexpressed in patients with Dupuytren contracture but not in those with normal fascia.[11]
A high number of mitochondria have been demonstrated in fibroblasts derived from diseased tissue, and a mitochondrial defect in the 16S ribosome RNA has been noted in 90% of affected patients compared with none in the control patients.[12] Thus, a mitochondrial etiology may be significant in some families, while a chromosomal defect may be important in others.
A number of growth factors, immunologic mediators, and free radicals are also implicated as causative factors, and it seems that an inciting factor triggers these mediators to stimulate myofibroblast proliferation.[13]
Diabetes mellitus has a strong association with Dupuytren contracture.[4][10][14][15][16] The prevalence of the disease in patients with diabetes ranges from 3% to 33% and increases with the duration of the diabetes. It is hypothesized that diabetes causes microvascular changes that produce local hypoxic tissue damage, inducing Dupuytren contracture. Patients with diabetes tend to have a mild form of the disease, with slow progression.
Greater alcohol intake is associated with an increased likelihood of Dupuytren contracture.[4][7][10][17] However, it should be noted that most patients with the disease do not misuse alcohol, and not all studies have found a significant association.[18] For unclear reasons, smoking increases risk, although the microvascular changes associated with smoking and the effect of carbon monoxide on mitochondrial cytochrome c, released by mitochondria in response to pro-apoptotic stimuli, may play a role.[17]
Trauma was first proposed to be a cause by Dupuytren himself. Since the first report that linked trauma to the disease, the strength of this association has been controversial. It is unclear why the disease would be caused by heavy labor, and further studies are required to control for smoking and alcohol use.[19]
There have been reports of a connection between epilepsy and Dupuytren contracture. When discontinuing anticonvulsant drugs, findings such as palmar cords and knuckle pad thickening have been reported to regress, although large cohort studies have not found an association with epilepsy or epilepsy medication and the disease. It would seem that epilepsy is not a risk for the disease, but anticonvulsant drugs (e.g., phenytoin and carbamazepine) may trigger it.[20]
Pathophysiology
Several hypotheses have been proposed for the underlying pathophysiologic mechanisms of Dupuytren contracture. Localized ischemia from diabetes, trauma, or other etiologies is thought to produce xanthine oxidase free radicals that may then damage the perivascular connective tissue and bring on a reparative response by surrounding fibroblasts, or triggering myofibroblasts. Fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-b) may signal an overproduction of myofibroblasts, leading to the formation of nodules and contractures. It has also been demonstrated that, in Dupuytren contracture, the proportions of the different types of collagen are altered, with collagen type 1 replaced by collagen type 3, similar to the proliferative phase of wound healing.
Classification
Characteristics of fibrous tissue deformity and contracture[2]
Three grades of Dupuytren contracture have been described, based on the characteristics of the fibrous tissue deformity and the presence of a contracture.
Grade 1: thickened nodule and a band in the palmar aponeurosis that may progress to skin tethering, puckering, or pitting.
Grade 2: peritendinous band with limited extension of the affected finger.
Grade 3: presence of flexion contracture. [Figure caption and citation for the preceding image starts]: Preoperative view of a small finger flexion contracture with surgical indicationsFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].
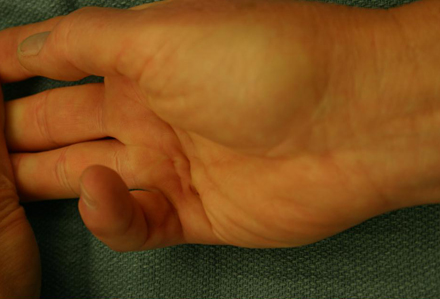 [Figure caption and citation for the preceding image starts]: Preoperative view of a small finger flexion contracture with surgical indicationsFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Preoperative view of a small finger flexion contracture with surgical indicationsFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].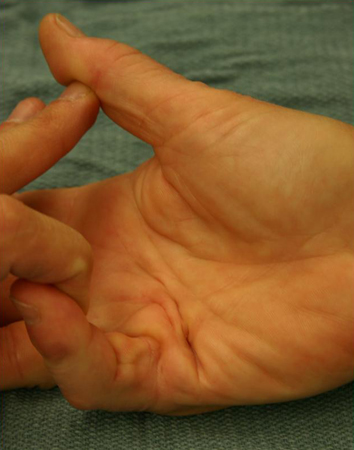
Presence of nodules and degree of contracture[3]
Seven stages of Dupuytren contracture have been described, based on the presence of nodules and the severity of the contracture.
Stage 0: no contracture
Stage N: no contracture, palpable nodule
Stage N/1: 0° to 5° contracture, palpable nodule
Stage 1: 6° to 45° contracture
Stage 2: 46° to 90° contracture
Stage 3: 91° to 135° contracture
Stage 4: >135° contracture
Use of this content is subject to our disclaimer