Approach
Diagnosis of heart failure with preserved ejection fraction (HFpEF) is challenging. The patient's history and risk factors may help, but imaging of the cardiovascular system and measurement of biomarkers are needed for a definitive diagnosis.[3][4]
According to the universal definition of heart failure (HF), the diagnosis requires presence of symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality and either elevated natriuretic peptide levels or objective evidence of pulmonary or systemic congestion, or both.[1]
History
The initial history should include an assessment of symptoms and the patient's functional capacity. Clinical symptoms and signs in HFpEF are often nonspecific, although the primary symptoms are breathlessness, fatigue, and fluid retention. Patients often find their exercise tolerance is limited by fatigue and breathlessness. Weight gain, peripheral edema, and abdominal congestion are common.[2][11][58] The New York Heart Association functional classification is used to describe the extent of a patient's symptoms. Patients with no symptoms are considered to be in class I. Patients with slight limitation of physical activity, such as dyspnea, fatigue, and palpitations, are considered to be in class II. Patients in class III have marked limitation of physical activity and patients in class IV have symptoms of heart failure at rest.[59]
Comorbidities and risk factors
A thorough history should be obtained in patients who present with signs and symptoms of heart failure, to identify comorbidities and risk factors for HFpEF (such as obesity, diabetes mellitus, hypertension, and renal insufficiency) as well as behaviors that might precipitate or accelerate the course of heart failure.
[Figure caption and citation for the preceding image starts]: Prevalence of individual chronic conditions in heart failure patients with preserved and reduced ejection fraction. Left panel, men; right panel, womenChamberlain AM, et al. Am J Med. 2015 Jan; 128(1): 38-45; used with permission [Citation ends].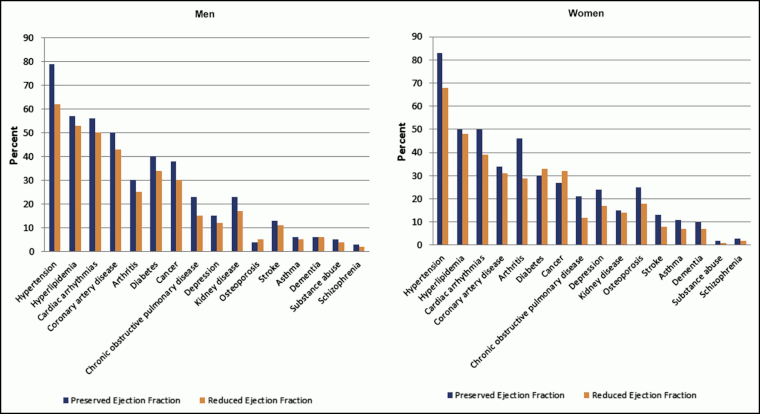
It is also important to consider potential HFpEF mimics (e.g., infiltrative cardiomyopathy, hypertrophic cardiomyopathy, valvular disease, pericardial disease), which may present with congestion, dyspnea, exercise intolerance, and for which there may be specific disease-directed therapy.[2][11]
Particular attention should also be made to the patient's diet, past and current medications, and habits (e.g., smoking, alcohol, illicit drug use).
Physical exam
Vital signs such as heart rate, blood pressure, respiration rate, and oxygen saturation should be measured and monitored. On physical examination, patients present with pulmonary rales, distended neck veins, a positive hepatojugular reflux, and lower extremity edema. An S3 or S4 gallop and/or hepatomegaly may be noted.
Score-based algorithms
Score-based algorithms for the diagnosis of HFpEF are available to help discriminate HFpEF from other causes of dyspnea.[60] The H₂FPEF score estimates the probability of HFpEF using obesity, atrial fibrillation, age >60 years, treatment with ≥2 antihypertensives, echocardiographic E/e’ ratio >9, and echocardiographic pulmonary artery systolic pressure >35 mmHg as predictive variables. Low (0 or 1) and high (6 to 9) scores indicate low and high likelihood of HFpEF, respectively, and those with an intermediate score may require additional evaluation and testing.[61] The HFA-PEFF diagnostic algorithm was developed by the Heart Failure Association of the European Society of Cardiology (ESC) and uses a stepwise approach.[62] Initially, a pretest assesses for heart failure symptoms and signs, typical clinical demographics (obesity, hypertension, diabetes, elderly, atrial fibrillation), and diagnostic laboratory tests (ECG and echocardiography). In the absence of overt noncardiac causes of dyspnea, HFpEF can be suspected if left ventricular ejection fraction (LVEF) is normal, there is no significant heart valve disease or cardiac ischemia, and there is at least one typical risk factor. The next step involves comprehensive echocardiography and serum natriuretic peptide levels from which a score is calculated that indicates probability of HFpEF. Those with an intermediate score may require additional functional testing.
The ESC heart failure guidelines acknowledge that, depending on which score is used, different patients will be referred for additional testing or diagnosed with HFpEF, and that not all centers will have access to the specialized tests recommended by these algorithms.[4] The ESC guidelines therefore recommend a simplified diagnostic approach based on symptoms and signs of heart failure, LVEF ≥50%, and objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of left ventricular diastolic dysfunction/raised left ventricular filling pressures, including raised natriuretic peptides.[4] The American College of Cardiology suggests that the H₂FPEF score may be more practical and useful in clinical practice than HFA-PEFF, with evidence of greater accuracy, but notes that a low H₂FPEF score in the context of HF symptoms and signs should not be used to exclude a diagnosis of HFpEF.[2]
Initial investigations
The first tests to perform in patients presenting with suspected heart failure are a 12-lead ECG, chest radiograph, blood tests, including natriuretic peptides (e.g., N-terminal prohormone B-natriuretic peptide [NT-proBNP]), and an echocardiogram. In patients with suspected HFpEF, an echocardiogram (including Doppler flow studies) should be performed to confirm or refute the diagnosis, categorize whether it is HFpEF or HFrEF, and exclude significant valvular, infiltrative myocardial and pericardial abnormalities. It should be noted that the presence of abnormal diastolic function on echocardiogram and elevated NT-proBNP levels are very useful in establishing a diagnosis of HFpEF, but in appropriate clinical circumstances their absence should not be used to exclude this diagnosis. Some patients with HFpEF may have normal BNP levels, or levels below the typical threshold for diagnosis.[63] In one study, normal BNP levels were present in 29% of symptomatic outpatients with HFpEF who had elevated pulmonary capillary wedge pressures.[64] Additionally, some patients may only have elevated pulmonary artery pressure with exercise, hence in these patients exercise hemodynamic assessment with right heart catheterization has been recommended to establish a diagnosis of HFpEF.[11]
ECG
12-lead ECG may reveal left ventricular (LV) hypertrophy, evidence of prior myocardial infarction, conduction defects, arrhythmias common to HFpEF such as atrial fibrillation, and low voltage suggestive of an infiltrative cardiomyopathy. It is very unusual for patients with heart failure to have a normal 12-lead ECG.
Blood tests
Serum electrolytes may show hypervolemic hyponatremia, which is common in severe heart failure due to expansion of extracellular volume. Potassium levels are usually normal, but use of thiazide diuretics may result in hypokalemia.
Renal function tests may be normal or may show increased creatinine and decreased estimated glomerular filtration rate (eGFR; e.g., in patients with long-standing hypertension, diabetes, or infiltrative diseases such as amyloidosis).
Complete blood count with iron levels (transferrin saturation) and ferritin is recommended.[3][4] Anemia can contribute to the patient's symptoms.
Blood glucose, thyroid function tests, and blood lipids are useful to assess for commonly associated comorbid disease.
Liver aminotransaminases (aspartate transaminase/alanine transaminase) may be elevated if there is liver congestion.
Natriuretic peptides are sensitive markers of heart failure and can help differentiate the etiology of dyspnea between heart failure and a noncardiac cause.[3][4] However, BNP alone is unable to show a distinction between HFpEF and HFrEF. Causes of elevated BNP other than heart failure include left ventricular hypertrophy, myocardial ischemia, tachycardia, right ventricular overload, hypoxemia (including from pulmonary embolism), renal dysfunction (eGFR <60 mL/minute), sepsis, COPD, diabetes, age >70 years, and cirrhosis. Causes of a low BNP include obesity and patients already treated with diuretics, ACE inhibitors, beta-blockers, or angiotensin-II receptor antagonists. Elevated BNP and NT-pro-BNP levels are associated with higher cardiovascular events in heart failure patients.[65] BNP levels are lower in patients with HFpEF than in patients with heart failure with reduced LVEF, but for a given BNP level, the prognosis in patients with HFpEF is as poor as in those with reduced LVEF.[66] Levels of BNP and NT-proBNP may be lower in patients with obesity; therefore, diagnostic sensitivity may be reduced in these patients.[2][3] Additionally, use of sacubitril/valsartan (angiotensin receptor-neprilysin inhibitor) may elevate levels of BNP, and use of NT-proBNP may be preferred in this case.[67]
Echocardiography
Transthoracic echocardiography can be used to establish the structure and function of the left ventricle and whether there is any other pathology that might explain the patients’ presentation, such as valvular disease or pericardial disease.[3][4]
Using echocardiography, it is possible to measure whether there is left ventricular hypertrophy (LV wall thickness is normally 7-12 mm) and/or left atrial dilatation. Patients with HFpEF usually have left atrial enlargement, elevated LV mass, and increased LV relative wall thickness.
Echocardiography can also be used to assess the filling pressures in the heart. The peak velocity across the mitral valve during early diastolic filling corresponds to the E wave. A is the transmitral flow during atrial contraction and corresponds to the A wave. The velocity of the E wave is determined by the balance between the rate of relaxation and the pressure gradient between the left atrium and the left ventricle in early diastole. In normal individuals, a typical E/A ratio is 1.0 to 1.5.
Doppler echocardiography is used to evaluate the characteristics of diastolic transmitral and pulmonary venous flow pattern that are used to assess diastolic function. Tissue Doppler imaging (TDI) seems to be less dependent on volume status compared with routine Doppler, and has been used to aid in the diagnosis of diastolic dysfunction.
TDI measures the change in velocity of the myocardial tissue itself during different phases of the cardiac cycle. Mitral annular velocity (E') has been used as a marker of diastolic function and is less preload-dependent than Doppler echocardiography.[68]
E/e' is the ratio of peak velocity across the mitral valve during early diastolic filling on routine Doppler echo and the mitral annular velocity on TDI. An E/e' ratio >15 is a reliable indicator of elevated left atrial pressure, whereas a ratio of <8 is generally found to be associated with normal filling pressure.[69]
Stage I or mild diastolic impairment: E/A ratio <1.0 due to impaired relaxation and significant contribution of atrial contraction to LV filling. Less filling of the LV in early diastole often results in enhanced contraction of the left atrium and an attendant increase in A velocity, reducing the E/A ratio. The E/e' ratio is generally normal.
Stage II or moderate diastolic impairment: E/A is normal, but E/A reversal (ratio <1.0) occurs with Valsalva maneuvers. Also called pseudo-normalization. This is due to reduced LV compliance, resulting in increased left atrial (LA) pressure. The E/e' ratio is intermediate-to-elevated in this situation.
Stage III or severe diastolic impairment: abnormal E/A ratio (>2.0), but changes with Valsalva maneuvers. Due to severe decrease in compliance, causing further increase in LA pressure. E/e' ratio is high (i.e., >15).
Stage IV (restrictive physiology): abnormal E/A ratio (>2.0) and remains fixed with Valsalva maneuvers. E/e' ratio is high (i.e., >15).
E- and A-wave velocities are affected by volume status, mitral valve dysfunction, and the presence of atrial fibrillation, so that their proper interpretation must take many of these factors into account.[69][Figure caption and citation for the preceding image starts]: Impaired relaxation: mitral inflow showing E/A reversalFrom the collection of Dr Jessica Webb; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Pseudonormalization of E/A mitral inflowFrom the collection of Dr Jessica Webb; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Pseudonormalization of E/A mitral inflowFrom the collection of Dr Jessica Webb; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Valsalva technique confirming impaired relaxationFrom the collection of Dr Marc Del Rosario [Citation ends].
[Figure caption and citation for the preceding image starts]: Valsalva technique confirming impaired relaxationFrom the collection of Dr Marc Del Rosario [Citation ends].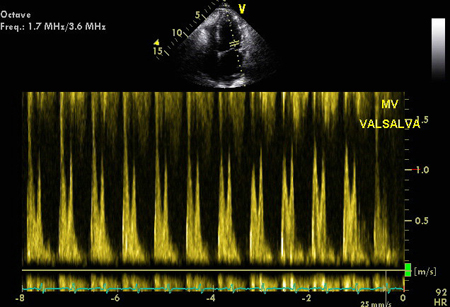 [Figure caption and citation for the preceding image starts]: Tissue Doppler imaging (TDI) of the basal LV showing increased E/E'From the collection of Dr Jessica Webb; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Tissue Doppler imaging (TDI) of the basal LV showing increased E/E'From the collection of Dr Jessica Webb; used with permission [Citation ends].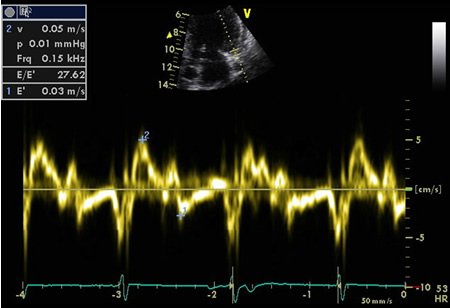 [Figure caption and citation for the preceding image starts]: Mitral inflow confirming restrictive fillingFrom the collection of Dr Jessica Webb; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Mitral inflow confirming restrictive fillingFrom the collection of Dr Jessica Webb; used with permission [Citation ends].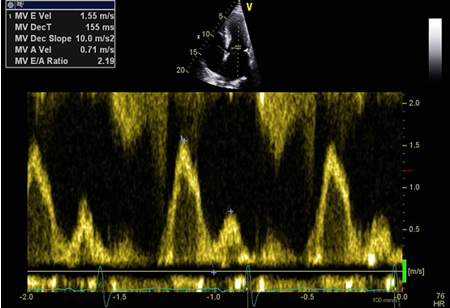
Chest x-ray
Chest x-ray may show cardiomegaly, pulmonary edema, or pleural effusions, or may identify other noncardiac causes of breathlessness; for example, pneumonia, oligemic lung field in pulmonary embolism, or collapse lung in pneumothorax.
Subsequent investigations
Cardiac imaging remains pivotal to the diagnosis of HFpEF. The choice of further investigations depends on local expertise. Practically, echocardiography is widely used, although it only offers an indirect assessment of LV filling with no characterization of myocardial tissue. Patients who exhibit chest pain with exertion or any symptom that is suspicious (e.g., an anginal equivalent) should be screened for CAD, as ischemia may contribute to abnormalities in systolic and diastolic function.
Cardiac magnetic resonance (CMR) imaging
CMR imaging represents the gold standard in quantification of systolic function and is increasingly used in the assessment of heart failure due to its unique, precise, noninvasive phenotypic characterization with high reproducibility and sufficient spatial and temporal resolution.[70]
CMR techniques to assess diastolic dysfunction can include measuring left atrial size, mitral inflow pattern, pulmonary vein assessment, LV time volume relations, LV myocardial tagging, and flow propagation velocity, calculating T1/myocardial fibrosis, and following injection of contrast. The below image shows a CMR 4-chamber image following injection of gadolinium contrast. In the late phase, normal myocardium should appear black (the valves appear white). In this image there is a subendocardial basal enhancement in the left ventricle that is consistent with amyloid infiltration. The next image is the same patient following contrast in the short axis at the basal level. In this image there is an almost complete white ring that represents the late gadolinium enhancement.[Figure caption and citation for the preceding image starts]: CMR of patient with cardiac amyloid infiltration. Following injection of gadolinium contrast, in the late phase there is subendocardial basal ring in the left ventricle (4-chamber view)From the collection of Dr Jessica Webb; used with permission [Citation ends].
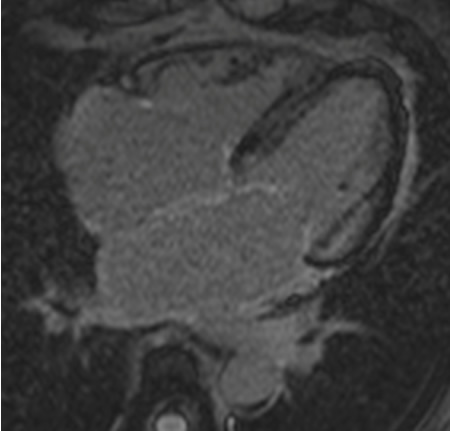 [Figure caption and citation for the preceding image starts]: CMR of patient with cardiac amyloid infiltration. Following injection of gadolinium contrast, in the late phase there is subendocardial basal ring in the left ventricle (short-axis basal image)From the collection of Dr Jessica Webb; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: CMR of patient with cardiac amyloid infiltration. Following injection of gadolinium contrast, in the late phase there is subendocardial basal ring in the left ventricle (short-axis basal image)From the collection of Dr Jessica Webb; used with permission [Citation ends].
CMR is also very useful for the diagnosis of pericardial disease causing heart failure (e.g., constrictive pericarditis).
Radionuclide imaging
Radionuclide ventriculography (MUGA scan) can provide an assessment of LVEF, although it cannot directly assess valvular abnormalities or cardiac hypertrophy.
Computed tomography coronary angiography (CTCA)
Other techniques such as computed tomography angiography CTCA may be considered in patients with suspected coronary artery disease (CAD) or pericardial disease.
Stress testing
Stress testing may be done using exercise (e.g., treadmill, bicycle) or pharmacologic (e.g., adenosine, dobutamine) methods. Exercise testing is preferred as it provides diagnostic and prognostic information from the patient's exercise capacity. Imaging techniques add to the sensitivity of the test. Depending on local expertise, patients can have stress echocardiography, perfusion CMR, or nuclear myocardial perfusion imaging with single photon emission computerized tomography.
Cardiopulmonary exercise testing (CPET) can help to confirm reduced exercise capacity and can also differentiate between cardiac and noncardiac causes of unexplained dyspnea.[3][4]
Cardiac catheterization
Cardiac catheterization and coronary angiography remain the diagnostic standard in diagnosing CAD. It may be performed in patients who have a high pretest likelihood of CAD and those who have a stress test showing ischemia.
An increase in pulmonary capillary wedge pressure (PCWP) of >15 mmHg at rest or >25 mmHg with exercise during right heart catheterization is considered diagnostic for heart failure.[11]
Other investigations for comorbidities and causes
Additional investigations for underlying causative factors and comorbidities e.g. hypertension, coronary artery disease, diabetes, atrial fibrillation, obesity, and renal dysfunction should be carried out and treated accordingly. See separate topics for details.
Use of this content is subject to our disclaimer