Most disorders are multifactorial in nature, and their evaluation is best approached by an interdisciplinary team, which may include a pediatrician for general and neurodevelopmental assessment, a pediatric gastroenterologist, a dietitian, a behavioral psychologist, a speech and language therapist, and an occupational therapist.[2]Borowitz KC, Borowitz SM. Feeding problems in infants and children: assessment and etiology. Pediatr Clin North Am. 2018 Feb;65(1):59-72.
http://www.ncbi.nlm.nih.gov/pubmed/29173720?tool=bestpractice.com
Diagnosis is usually made on clinical grounds. In the presence of a normal clinical exam, with normal neurodevelopmental assessment and normal anthropometric measurements, diagnostic tests are rarely needed.
General history
Prenatal history should be reviewed, checking for results of the prenatal ultrasound scan and signs of possible neuromuscular problems (e.g., polyhydramnios, decreased fetal movements). Prematurity and intrauterine growth restriction increase the risk of subsequent feeding disorders, and the gestational age, birth weight, subsequent weight gain, and measurements of linear growth (length and head circumference) should be reviewed carefully. Perinatal events such as time taken to pass meconium and initial feeding regimen (type of milk, duration of feeding, feeding interval) should be elicited.
It is important to review growth from birth and relate this to any changes in feeding. For example, infants with celiac disease may not gain weight following weaning.
Evidence of previous illnesses and hospitalizations should be sought. Concurrent illness may impact on an infant's ability to feed (e.g., poor respiratory reserve in patients with congenital heart disease or chronic lung disease). Medical interventions such as tracheal intubation or nasogastric feeding are postulated to increase sensory aversion to feeding.[3]Rommel N, De Meyer AM, Feenstra L, et al. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003 Jul;37(1):75-84.
http://www.ncbi.nlm.nih.gov/pubmed/12827010?tool=bestpractice.com
Problems with feeding may also be the presenting complaint of more complex conditions such as cerebral palsy, which become apparent only as the infant matures. Regular hospital attendance can also be evidence of stress within a family, which can result from or lead to difficulties with feeding.
Elicit the infant’s surgical history, to include correction of oropharyngeal and gastrointestinal (GI) tract abnormalities, GI tract resection, stoma formation, and neonatal cardiac surgery.
Enquire about symptoms of:
Vomiting
Abdominal pain, distension, or colic
Constipation or diarrhea
Postural changes during feeds
Respiratory problems
Atopy.
Recurrent vomiting with a normal physical exam and normal growth is common in uncomplicated GERD.[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
Vomiting associated with abdominal pain, colic, and constipation may be seen in both GERD and cows' milk protein allergy (CMPA). A sudden onset of vomiting in a previously well child may be a symptom of other conditions, notably infections such as meningitis and urinary tract infection, which should be considered and ruled out first. Bilious vomiting in a term infant indicates an upper GI obstruction usually requiring surgical intervention. However, this is a common finding during the establishment of enteral feeding in premature neonates, and is normally managed medically in this population. Simple regurgitation without any other symptoms is physiologic and does not require either investigation or treatment.[36]National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease in children and young people: diagnosis and management. October 2019 [internet publication].
https://www.nice.org.uk/guidance/ng1
[37]Baird DC, Harker DJ, Karmes AS. Diagnosis and treatment of gastroesophageal reflux in infants and children. Am Fam Physician. 2015 Oct 15;92(8):705-14.
https://www.aafp.org/afp/2015/1015/p705.html
http://www.ncbi.nlm.nih.gov/pubmed/26554410?tool=bestpractice.com
Colic is defined as paroxysms of irritability or crying lasting >3 hours per day and occurring >3 days per week.[38]Wessel MA, Cobb JC, Jackson EB, et al. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954 Nov;14(5):421-35.
http://www.ncbi.nlm.nih.gov/pubmed/13214956?tool=bestpractice.com
Colic can occur in isolation, or concomitantly with GERD or CMPA.[39]Miller-Loncar C, Bigsby R, High P, et al. Infant colic and feeding difficulties. Arch Dis Child. 2004 Oct;89(10):908-12.
http://www.ncbi.nlm.nih.gov/pubmed/15383432?tool=bestpractice.com
Infants who are reported to have symptoms of colic have more disorganised feeding behaviors, less rhythmic sucking, and lower responsiveness during feeds.[39]Miller-Loncar C, Bigsby R, High P, et al. Infant colic and feeding difficulties. Arch Dis Child. 2004 Oct;89(10):908-12.
http://www.ncbi.nlm.nih.gov/pubmed/15383432?tool=bestpractice.com
Coughing or retching at mealtimes may indicate difficulties with swallowing and possible aspiration.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Chronic aspiration can lead to recurrent pneumonia, even in the absence of these symptoms; this is particularly the case if the infant is neurologically impaired.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
[40]Bernard-Bonnin AC. Feeding problems of infants and toddlers. Can Fam Physician. 2006 Oct;52(10):1247-51.
https://www.cfp.ca/content/52/10/1247.full.pdf
http://www.ncbi.nlm.nih.gov/pubmed/17279184?tool=bestpractice.com
[41]Arvedson JC. Feeding children with cerebral palsy and swallowing difficulties. Eur J Clin Nutr. 2013 Dec;67 (Suppl 2):S9-12.
https://www.nature.com/articles/ejcn2013224
http://www.ncbi.nlm.nih.gov/pubmed/24301008?tool=bestpractice.com
Recurrent cough, wheeze, stridor, or hoarseness of cry can be associated with GERD.[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
Apnea and bradycardia with feeds may reflect either a central problem with breathe-suck-swallow coordination, or may reflect GERD.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Increased respiratory rate and increased work of breathing at rest will impair ability to feed.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Poor coordination, weak suck, and short sucking bursts are described in infants with severe bronchopulmonary dysplasia.[24]Mizuno K, Nishida Y, Taki M, et al. Infants with bronchopulmonary dysplasia suckle with weak pressures to maintain breathing during feeding. Pediatrics. 2007 Oct;120(4):e1035-42.
http://www.ncbi.nlm.nih.gov/pubmed/17893188?tool=bestpractice.com
Posture changes during feeds may indicate discomfort.[2]Borowitz KC, Borowitz SM. Feeding problems in infants and children: assessment and etiology. Pediatr Clin North Am. 2018 Feb;65(1):59-72.
http://www.ncbi.nlm.nih.gov/pubmed/29173720?tool=bestpractice.com
Arching the neck and turning the head to one side may be seen in Sandifer syndrome, an uncommon manifestation of severe GERD.
Rashes (especially eczema), rhinitis, diarrhea, and constipation may be symptoms of CMPA.[42]Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221-9.
https://journals.lww.com/jpgn/Fulltext/2012/08000/Diagnostic_Approach_and_Management_of_Cow_s_Milk.28.aspx
http://www.ncbi.nlm.nih.gov/pubmed/22569527?tool=bestpractice.com
Watery stool, abdominal distension, and flatulence suggest lactose intolerance.
Family history of atopy or feeding problems should be sought. Families with atopy, particularly those with CMPA, are more likely to have children affected with conditions such as CMPA and eosinophilic esophagitis.[43]De Greef E, Hauser B, Devreker T, et al. Diagnosis and management of cow's milk protein allergy in infants. World J Pediatr. 2012 Feb;8(1):19-24.
http://www.ncbi.nlm.nih.gov/pubmed/22282379?tool=bestpractice.com
A familial pattern is seen in pyloric stenosis and in celiac disease.[44]Galea R, Said E. Infantile hypertrophic pyloric stenosis: an epidemiological review. Neonatal Netw. 2018 Jul;37(4):197-204.
http://www.ncbi.nlm.nih.gov/pubmed/30567916?tool=bestpractice.com
[45]Singh P, Arora S, Lal S, et al. Risk of celiac disease in the first- and second-degree relatives of patients with celiac disease: a systematic review and meta-analysis. Am J Gastroenterol. 2015 Nov;110(11):1539-48.
http://www.ncbi.nlm.nih.gov/pubmed/26416192?tool=bestpractice.com
The risk of feeding disorders is increased if an infant’s parents had feeding problems in their own infancy.[46]Dahl M, Eklund G, Sundelin C. Early feeding problems in an affluent society. II. Determinants. Acta Paediatr Scand. 1986 May;75(3):380-7.
http://www.ncbi.nlm.nih.gov/pubmed/3460308?tool=bestpractice.com
Feeding history and observation of feed
A detailed feeding history should be sought, ideally with the help of a pediatric dietitian. Feeding history should include:[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Diet since birth
Amount of feed (looking for excessive or inadequate intake, errors in the preparation of formula feeds)
Types of feed (including changes in formula, and introduction of solids)
Feeding interval
Time taken to feed. Efficient feeding usually takes <30 minutes per feed.
Where breastfeeding is the exclusive mode of feeding, details about latching on, awareness of milk supply, time spent feeding, and nipple pain should be elicited. Breastfeeds commonly take 15 to 20 minutes, although can range from 10 to 45 minutes on some occasions.
Age at weaning and amount of solids taken during a day should be ascertained in older infants, because decreased solid intake has been associated with faltering growth in this group.[47]Emond A, Drewett R, Blair P, et al. Postnatal factors associated with failure to thrive in term infants in the Avon longitudinal study of parents and children. Arch Dis Child. 2007 Feb;92(2):115-9.
http://www.ncbi.nlm.nih.gov/pubmed/16905563?tool=bestpractice.com
Feeding should be observed for a full 20 minutes to gain an accurate impression of the infant's feeding pattern.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
The infant should be observed to assess response to cues, level of infant alertness, breathe-suck-swallow coordination, and quality of feed (length, quantity, associated symptoms). The interactions between the caregiver and the infant should be observed, looking for positive interactions (maintaining eye contact, offering praise for good behaviors, reciprocal vocalization, responding to satiety cues) and negative interactions (forced feeding, bribing, distracting).[40]Bernard-Bonnin AC. Feeding problems of infants and toddlers. Can Fam Physician. 2006 Oct;52(10):1247-51.
https://www.cfp.ca/content/52/10/1247.full.pdf
http://www.ncbi.nlm.nih.gov/pubmed/17279184?tool=bestpractice.com
Behavioral and social issues
A behavioral component is found in 80% of feeding disorders, and is the primary cause of the feeding disorder in 10% of cases.[3]Rommel N, De Meyer AM, Feenstra L, et al. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003 Jul;37(1):75-84.
http://www.ncbi.nlm.nih.gov/pubmed/12827010?tool=bestpractice.com
[4]Burklow KA, Phelps AN, Schultz JR, et al. Classifying complex paediatric feeding disorders. J Pediatr Gastroenterol Nutr. 1998 Aug;27(2):143-7.
http://www.ncbi.nlm.nih.gov/pubmed/9702643?tool=bestpractice.com
Diagnosis is primarily obtained from the history (food aversion, food refusal, stressful mealtimes). A feed diary documenting these symptoms may be extremely useful in the initial assessment of an infant with a feeding disorder.
An observed feed over 20 minutes to assess caregiver-infant interactions supports the diagnosis. Positive interactions include positive reinforcement for accepting food, good eye contact, and praise. Negative interactions include attempting to force the infant to feed, coaxing the infant, or using distraction techniques during mealtimes.[6]Ramsay M, Gisel E, Boutry M. Non-organic failure to thrive: growth failure secondary to feeding-skills disorder. Dev Med Child Neurol. 1993 Apr;35(4):285-97.
http://www.ncbi.nlm.nih.gov/pubmed/8335143?tool=bestpractice.com
[48]Piazza CC. Feeding disorders and behaviour: what have we learned? Dev Disabil Res Rev. 2008;14(2):174-81.
http://www.ncbi.nlm.nih.gov/pubmed/18646017?tool=bestpractice.com
Overall responsiveness and temperament of the infant should be assessed as well.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Normal infants can cry up to an average of 2 hours per day, and the feed diary may be helpful to determine the length of irritability and any temporal association with feeds.
An understanding of any stresses or mental illness within the family will be helpful both in making the initial diagnosis and in optimizing its management. Feeding disorders in infants have been associated with depression in adult caregivers.[49]Wright CM, Parkinson KN, Drewett RF. The influence of maternal socioeconomic and emotional factors on infant weight gain and weight faltering (failure to thrive): data from a prospective birth cohort. Arch Dis Child. 2006 Apr;91(4):312-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2065961
http://www.ncbi.nlm.nih.gov/pubmed/16397011?tool=bestpractice.com
Caregivers may report stressful mealtimes due to the length of time taken to feed, behavior of the child during feeding, or difficulty in giving adequate amounts or variety of food.[7]Milnes SM, Piazza CC, Carroll-Hernandez TA. Assessment and treatment of pediatric feeding disorders. September 2013 [internet publication].
https://www.child-encyclopedia.com/pdf/expert/child-nutrition/according-experts/assessment-and-treatment-pediatric-feeding-disorders
[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
[41]Arvedson JC. Feeding children with cerebral palsy and swallowing difficulties. Eur J Clin Nutr. 2013 Dec;67 (Suppl 2):S9-12.
https://www.nature.com/articles/ejcn2013224
http://www.ncbi.nlm.nih.gov/pubmed/24301008?tool=bestpractice.com
An early feeding disorder can be compounded by abnormal learned behavioral responses to these stresses.[7]Milnes SM, Piazza CC, Carroll-Hernandez TA. Assessment and treatment of pediatric feeding disorders. September 2013 [internet publication].
https://www.child-encyclopedia.com/pdf/expert/child-nutrition/according-experts/assessment-and-treatment-pediatric-feeding-disorders
Clinical exam
Careful measurements of weight, length, and head circumference should be plotted on appropriate charts and compared with growth from birth.
The symmetry of the infant's face and jaw, lips, and palate, and the rhythm and strength of non-nutritive and feeding suck should be assessed to rule out craniofacial abnormalities such as Pierre Robin sequence.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
An open-mouthed posture may reflect nasal or pharyngeal obstruction.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Tonsillar hypertrophy should be considered if mouth breathing or stertorous (snoring) breathing is heard.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Tongue-tie has been associated with suckling difficulties in breastfed infants.[28]Messner AH, Walsh J, Rosenfeld RM, et al. Clinical consensus statement: ankyloglossia in children. Otolaryngol Head Neck Surg. 2020 May;162(5):597-611.
https://journals.sagepub.com/doi/10.1177/0194599820915457
http://www.ncbi.nlm.nih.gov/pubmed/32283998?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Infant with tongue-tie (ankyloglossia)Shutterstock [Citation ends]. [Figure caption and citation for the preceding image starts]: An infant with Pierre Robin sequenceReproduced from https://pubmed.ncbi.nlm.nih.gov/22300418/ under a CC BY 2.0 license; no changes have been made to the image [Citation ends].
[Figure caption and citation for the preceding image starts]: An infant with Pierre Robin sequenceReproduced from https://pubmed.ncbi.nlm.nih.gov/22300418/ under a CC BY 2.0 license; no changes have been made to the image [Citation ends].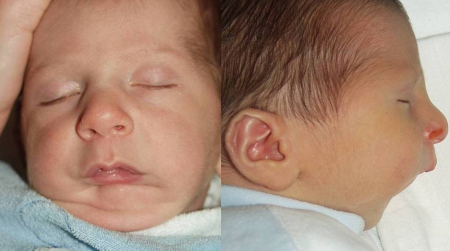
Rashes (especially eczema) may be a sign of CMPA.[42]Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221-9.
https://journals.lww.com/jpgn/Fulltext/2012/08000/Diagnostic_Approach_and_Management_of_Cow_s_Milk.28.aspx
http://www.ncbi.nlm.nih.gov/pubmed/22569527?tool=bestpractice.com
Neurodevelopmental assessment is of particular importance. Feeding disorders are seen in up to 80% of children with neurodevelopmental delay.[3]Rommel N, De Meyer AM, Feenstra L, et al. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003 Jul;37(1):75-84.
http://www.ncbi.nlm.nih.gov/pubmed/12827010?tool=bestpractice.com
Failure to achieve feeding milestones may be the presenting sign of more global developmental delay. Attention should be paid to the infant's posture, position during feeds, truncal tone, and movement. Drooling may be a sign of difficulty swallowing. The infant's response to sensory stimuli may also give more clues about developmental issues.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
Tests
Often feeding problems can be diagnosed from history, feeding assessment, and clinical exam, and no tests are required. Conditions that can be diagnosed clinically include simple overfeeding, prematurity, GERD, behavioral issues, and some craniofacial, neurologic, and genetic conditions. Investigations may be required to assess for complications and to guide management.
Trial of dietary changes
Diagnosis of CMPA is confirmed by exclusion of cows’ milk from the diet for 2 to 4 weeks, followed by a rechallenge with cows milk protein after resolution of symptoms. Recurrence of symptoms after the cows’ milk challenge confirms the diagnosis. If symptoms fail to improve with dietary modification, the original diagnosis should be reconsidered.[42]Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221-9.
https://journals.lww.com/jpgn/Fulltext/2012/08000/Diagnostic_Approach_and_Management_of_Cow_s_Milk.28.aspx
http://www.ncbi.nlm.nih.gov/pubmed/22569527?tool=bestpractice.com
Lactose intolerance is diagnosed by performing a therapeutic trial of lactose-free feeds. Hypoallergenic formula preparations are available that are suitable for use in infants with either lactose or cows' milk intolerance, which is helpful given the overlap of symptoms in these disorders.
Laboratory tests
Symptomatic infants with suspected celiac disease should be screened by measuring immunoglobulin A (IgA) antibodies against tissue transglutaminase and total serum IgA.[50]Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020 Jan;70(1):141-56.
https://journals.lww.com/jpgn/Fulltext/2020/01000/European_Society_Paediatric_Gastroenterology,.24.aspx
http://www.ncbi.nlm.nih.gov/pubmed/31568151?tool=bestpractice.com
Radioallergosorbent testing to cows’ milk protein or skin prick testing can support the diagnosis of CMPA, but must be interpreted in the context of the patient’s history and cows’ milk challenge response. Negative test results do not exclude CMPA.[42]Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221-9.
https://journals.lww.com/jpgn/Fulltext/2012/08000/Diagnostic_Approach_and_Management_of_Cow_s_Milk.28.aspx
http://www.ncbi.nlm.nih.gov/pubmed/22569527?tool=bestpractice.com
A fresh stool sample can be tested for fecal-reducing substances in suspected lactose intolerance. A positive result confirms the diagnosis.
Chest x-ray
Performed when aspiration is suspected. Chronic aspiration can lead to recurrent pneumonia, even in the absence of any symptoms of regurgitation or coughing; this is particularly the case if the infant is neurologically impaired.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
[40]Bernard-Bonnin AC. Feeding problems of infants and toddlers. Can Fam Physician. 2006 Oct;52(10):1247-51.
https://www.cfp.ca/content/52/10/1247.full.pdf
http://www.ncbi.nlm.nih.gov/pubmed/17279184?tool=bestpractice.com
Upper GI contrast study
Used to confirm or exclude anatomic abnormalities.[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
Videofluoroscopic swallowing assessment
This test provides dynamic imaging of oral, pharyngeal, and upper esophageal swallowing phases.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
It can provide information about strength and coordination of muscles in the oropharynx, as well as presence and timing of aspiration. This information is useful to determine optimal feeding position, rate of feeding, and food texture.
Fiberoptic endoscopic evaluation of swallowing with sensory testing (FESST)
FESST may be useful to determine pharyngeal swallowing function. It does not provide visualization of the oral phase of swallowing, because the endoscope is passed transnasally.[8]Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118-27.
http://www.ncbi.nlm.nih.gov/pubmed/18646015?tool=bestpractice.com
[25]Delaney AL, Arvedson JC. Development of swallowing and feeding: prenatal through first year of life. Dev Disabil Res Rev. 2008;14(2):105-17.
http://www.ncbi.nlm.nih.gov/pubmed/18646020?tool=bestpractice.com
FESST is radiation-free and is generally well tolerated, and can be repeated if needed. It is a highly specialized test, usually available only in tertiary centers.
Upper GI endoscopy
Referral for upper GI endoscopy and biopsy should be considered for children with GERD if there is feeding aversion and a history of regurgitation.[36]National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease in children and young people: diagnosis and management. October 2019 [internet publication].
https://www.nice.org.uk/guidance/ng1
This test can distinguish between GERD and eosinophilic esophagitis. Duodenal biopsy can confirm a diagnosis of celiac disease.
Esophageal 24-hour pH study/combined esophageal pH and impedance monitoring
Esophageal pH study or combined esophageal pH and impedance monitoring can be helpful to correlate symptoms with episodes of acid reflux.[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
Accurate parental reporting of symptoms is crucial.[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
Esophageal pH studies cannot detect reflux episodes with pH >4.[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
Impedance monitoring detects episodes of gaseous or liquid reflux by monitoring distension of distal esophagus. This test should be considered in patients with associated neurologic or neuromuscular problems, suspected recurrent aspiration pneumonia, or unexplained apneas.[36]National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease in children and young people: diagnosis and management. October 2019 [internet publication].
https://www.nice.org.uk/guidance/ng1
Impedance monitoring can detect nonacidic reflux (pH ≥4).[35]Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958910
http://www.ncbi.nlm.nih.gov/pubmed/29470322?tool=bestpractice.com
It may be particularly helpful to detect reflux in premature and very young infants where feeds are frequent and nonacidic reflux is more common.[15]Lopez-Alonso M, Moya MJ, Cabo JA, et al. Twenty-four hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics. 2006 Aug;118(2):e299-308.
http://www.ncbi.nlm.nih.gov/pubmed/16831894?tool=bestpractice.com
Other specialized investigations
Other tests such as genetics assessment or echocardiography will be determined by the suspected clinical syndrome. Many patients with complex needs, such as infants with cleft lip and palate, will have been under the review of multidisciplinary teams since birth.[51]Bessell A, Hooper L, Shaw WC, et al. Feeding interventions for growth and development in infants with cleft lip, cleft palate or cleft lip and palate. Cochrane Database Syst Rev. 2011 Feb 16;(2):CD003315.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003315.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/21328261?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: An infant with Pierre Robin sequenceReproduced from https://pubmed.ncbi.nlm.nih.gov/22300418/ under a CC BY 2.0 license; no changes have been made to the image [Citation ends].
[Figure caption and citation for the preceding image starts]: An infant with Pierre Robin sequenceReproduced from https://pubmed.ncbi.nlm.nih.gov/22300418/ under a CC BY 2.0 license; no changes have been made to the image [Citation ends].