Approach
The diagnosis of a melanocytic nevus can often be made by history and physical exam alone. There are diagnostic tests (dermatoscopy, total body photography, non-invasive imaging with computer-generated algorithms, and biopsy) that can be used to increase the diagnostic accuracy. Dermatoscopy or epiluminescence microscopy may be a useful adjunct to physical exam for a trained practitioner when deciding whether the nevus in question is a melanoma. Additionally, spectrophotometric intracutaneous analysis involves creating computer-generated algorithms with newer non-invasive hand-held devices, which use light scatter to help medical practitioners predict the disorganization, and thus likelihood of malignancy, of a lesion. Biopsy is also required if there is clinical suspicion of melanoma.
History
The history of the lesion in question is an integral part of determining the diagnosis of a melanocytic nevus. Determining whether the lesion was present at birth or shortly thereafter will point toward the diagnosis of a congenital nevus. The differentiation of benign from malignant is the main medical concern when discussing melanocytic nevi; therefore, when asking about nevi that are changing or are newly acquired, questions should include whether the nevus is changing color or has developed multiple colors; whether there has been relatively rapid growth with change in size, shape, or surface, or easy bleeding; and the "ABCDEs" of melanoma:[27][28]
A: asymmetry
B: border irregularity
C: color variegation
D: diameter >6 mm
E: evolution.
Itching, burning, or pain may or may not be additional clues that alert the physician to the possibility of melanoma, but most patients with melanoma are asymptomatic.[24]
Physical exam
A full body skin exam is an essential component in diagnosing melanocytic nevi and differentiating benign from malignant pigmented lesions.[24] Patients often are unaware of skin lesions on areas that are not readily visible.
Common acquired nevi
These have a wide range of clinical appearances but tend to be symmetric, evenly pigmented, round to oval, evenly bordered, and homogeneous.[7] They often appear as flat, brown macules, but also can be dome-shaped, mamillated, pink-to-brown papules. [Figure caption and citation for the preceding image starts]: A common acquired nevusFrom the collection of Laurel Schwartz, Thomas Jefferson University [Citation ends].
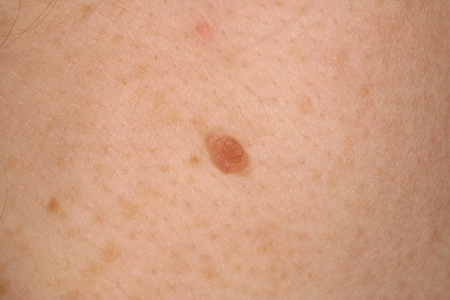 [Figure caption and citation for the preceding image starts]: A common acquired nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: A common acquired nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].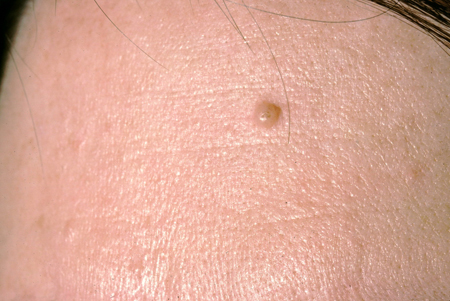
Small congenital nevi
These can be clinically indistinguishable from common acquired nevi.[6] Small-to-medium congenital nevi are often round-to-oval, brown-to-tan macules or papules with a well-defined border, and may have prominent hair. Large congenital nevi are often located on the posterior trunk, can be sizable, and may have satellite lesions. [Figure caption and citation for the preceding image starts]: A small congenital melanocytic nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
 [Figure caption and citation for the preceding image starts]: A medium congenital nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: A medium congenital nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].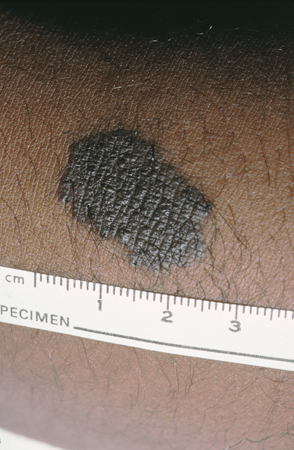 [Figure caption and citation for the preceding image starts]: A giant congenital melanocytic nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: A giant congenital melanocytic nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].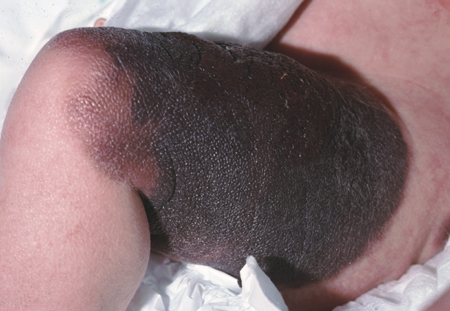
Nevus spilus
These are light brown patches with darker brown macules or papules studded within. Diagnosis is often made on clinical findings alone.[7][29][Figure caption and citation for the preceding image starts]: A nevus spilusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
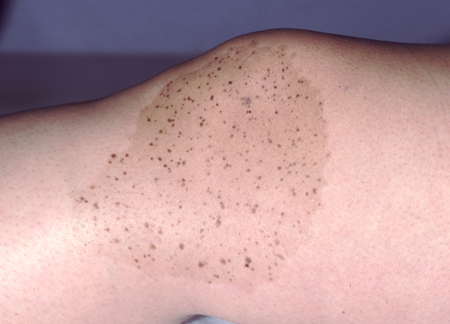
Blue nevi
These are gray-blue to blackish small macules and papules, often found on the dorsal extremities, sacrum, and head and neck.[1][5][7][Figure caption and citation for the preceding image starts]: A blue nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
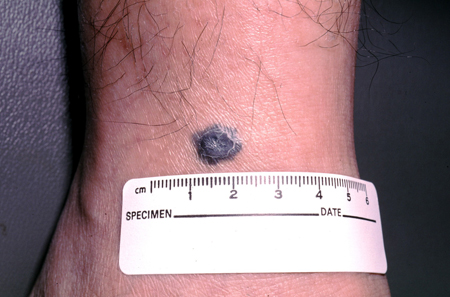 [Figure caption and citation for the preceding image starts]: A blue nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: A blue nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].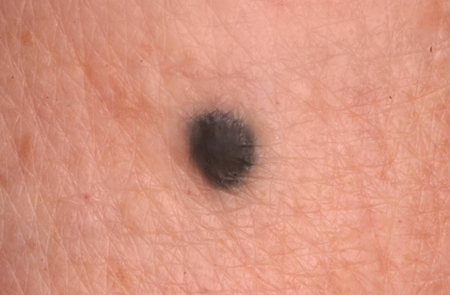
Halo nevi
These contain a nevus of varying morphology with a halo of depigmentation.[7] The nevus often regresses after a period of time, leaving a depigmented macule, which may eventually repigment over time. They are often found on the upper back in young adults and children, and are associated with an increased number of nevi and with a personal or family history of vitiligo.[5][7] Diagnosis is often made on clinical findings alone. [Figure caption and citation for the preceding image starts]: A halo nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].

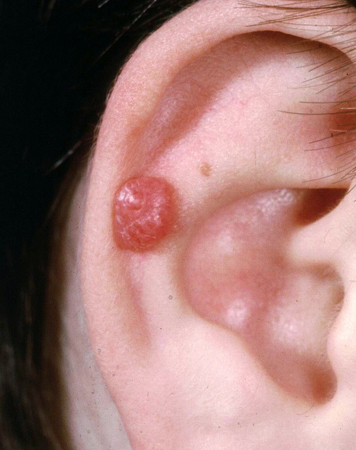
Spitz nevi
These are uniformly pink, tan, red, or red-brown solitary dome-shaped papules.[7] Spitz nevi tend to be found on the head and neck, and can be solitary or, rarely, grouped.[5] A darkly pigmented variant, also known as pigmented spindle cell nevus (of Reed), is typically found on the trunk and extremities.[5] Pigmented Spitz nevi are small (2 to 5 mm), flat, and dark-brown to jet-black in color, rather than dome-shaped and reddish in color. [Figure caption and citation for the preceding image starts]: A Spitz nevus on the earFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
 [Figure caption and citation for the preceding image starts]: A pigmented Spitz nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: A pigmented Spitz nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].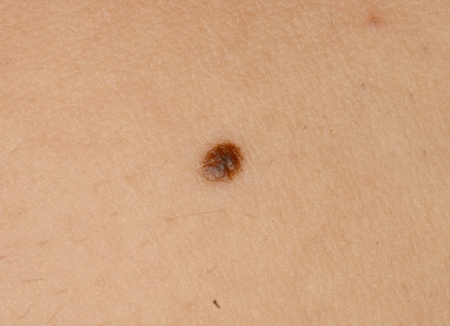
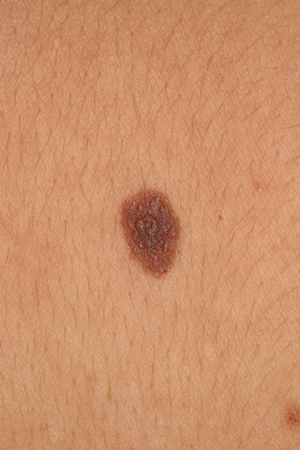
Atypical, dysplastic, or Clark nevi
These are designations that have been used to describe a type of nevus that shares some clinical features of melanoma, including the ABCDE criteria (asymmetry of the lesion, border irregularity or lack of circumscription, color variegation, diameter >6 mm, and evolution), but to a lesser degree.[7][17][30][31][32] Typically, they are >5 mm, flat or slightly elevated, variegated in color, and irregular in border. The lesion varies from tan to dark brown in color, but may have hypopigmented and pink areas. [Figure caption and citation for the preceding image starts]: A dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
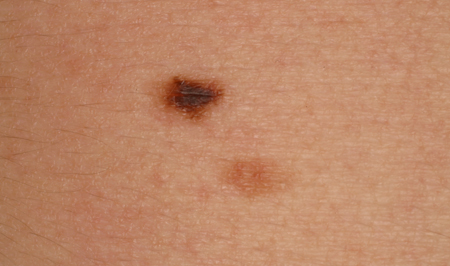 [Figure caption and citation for the preceding image starts]: A dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: A dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].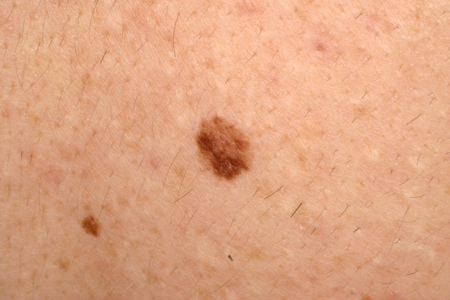
In patients with many nevi, some practitioners advocate the "ugly duckling" sign.[33] This method utilizes pattern recognition to identify lesions that are outliers to the patient's typical nevi. For example, if a patient has multiple nevi that fulfill the ABCDE criteria, using the patient's own nevi as a control to find the one that is dissimilar to the rest can help practitioners in screening for melanoma.
Wiesner nevus, deep penetrating nevus, and pigmented epithelioid melanocytoma are rare melanocytic benign neoplasms with distinctive histopathological features and mutations. They are usually combined nevi that consists of two different populations of melanocytes. One population usually harbors a mutation in BRAF while the other population, which characterizes the nevus, harbors a mutation that is particular to the respective nevus.
Wiesner nevus (BAP1-deficient nevus)
Wiesner nevus has similar clinical features to common acquired nevus or small congenital nevus. The population that characterizes the nevus consists of large epithelioid melanocytes that have cytologic features of a Spitz nevus and therefore are often misinterpreted as such. The epithelioid component harbors the mutation in BRCA1 associated-protein 1 (BAP1). The vast majority of Wiesner nevus is the result of sporadic somatic mutations in BAP1. Those who harbor the rare germline mutation in BAP1 have an increased risk of developing mesothelioma, uveal and cutaneous melanomas, and renal-cell carcinoma.[34]
Deep penetrating nevus
Also known as plexiform spindle cell nevus, deep penetrating nevus is a rare intradermal nevus that usually presents in young adults as bluish dark brown to black papule that may simulate a melanoma.[35]
Pigmented epithelioid melanocytoma
Pigmented epithelioid melanocytoma usually presents as a darkly pigmented papule or nodule in children and young adults. Previous designations include animal-type melanoma and epithelioid blue nevus. These lesions are known to occur in patients with Carney complex. According to the World Health Organization (WHO) classification, they are classified as intermediate-grade melanocytic lesions partly due to frequent involvement of the lymph nodes. As a rare melanocytic neoplasm, long-term follow-up of a large number of cases does not exist. The small number of cases that have limited follow-up indicates a benign course.[36]
Dermatoscopy
This technique can be performed to increase diagnostic accuracy in a trained clinician when deciding whether the lesion in question is a melanocytic nevus or a melanoma (or other neoplasm).[37] Also called epiluminescence microscopy, it permits visualization of pigmented lesions in vivo. A dermatoscope renders the cornified layer translucent, resulting in better visualization of the subsurface structures within the viable epidermis and the superficial dermis. The color of the lesion can indicate the location of the pigment in the skin: gray-blue indicates melanin in the dermis, as in a blue nevus; light or dark brown indicates melanin in the dermal-epidermal junction and horny layer, as in acquired nevi. The presence of a pigment network, clustered globules, and dots may favor a benign melanocytic nevus.
Dermatoscopy may be a useful adjunct to physical exam in a trained practitioner when the melanocytic nevus clinically has some features of the ABCDE mnemonic. Because these criteria are subjective and vary only in matter of degree between dysplastic nevi and melanoma, it can often be a diagnostic challenge to the physician to distinguish the two.[17][32] For these questionable lesions, dermatoscopic photographs allow detection of subtle changes over a short period of time, which may provide a better clue as to the nature of the lesion. A large repository of dermatoscopic images has been created to develop artificial intelligence in the future to assist in managing melanocytic lesions.[38][Figure caption and citation for the preceding image starts]: Dermatoscopy of a blue nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].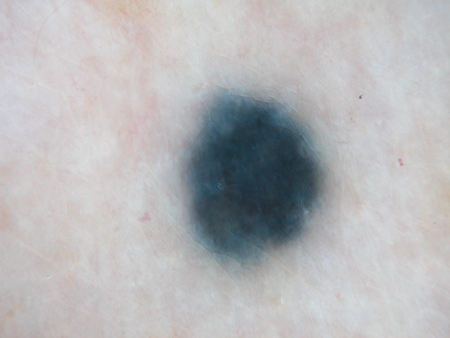 [Figure caption and citation for the preceding image starts]: Dermatoscopy of a nevus with a reticular pigment networkFrom the collection of Laurel Schwartz, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: Dermatoscopy of a nevus with a reticular pigment networkFrom the collection of Laurel Schwartz, Thomas Jefferson University [Citation ends].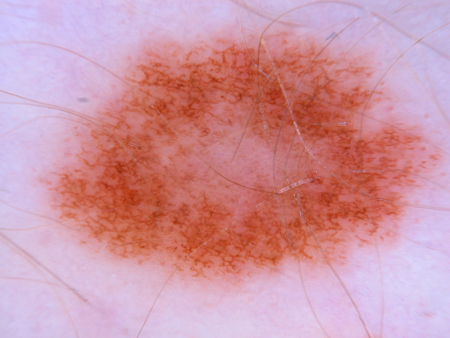 [Figure caption and citation for the preceding image starts]: Dermatoscopy of a cobblestone pattern with a peripheral pigment networkFrom the collection of Jason B. Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: Dermatoscopy of a cobblestone pattern with a peripheral pigment networkFrom the collection of Jason B. Lee, Thomas Jefferson University [Citation ends].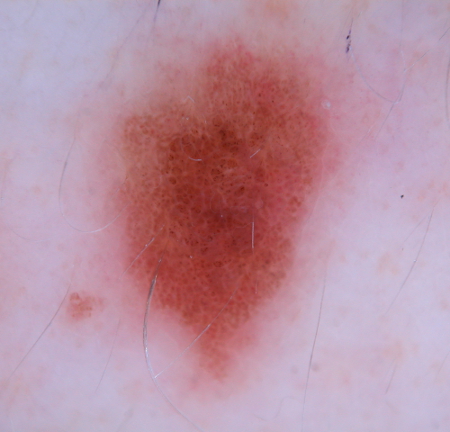 [Figure caption and citation for the preceding image starts]: Dermatoscopy of a dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: Dermatoscopy of a dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].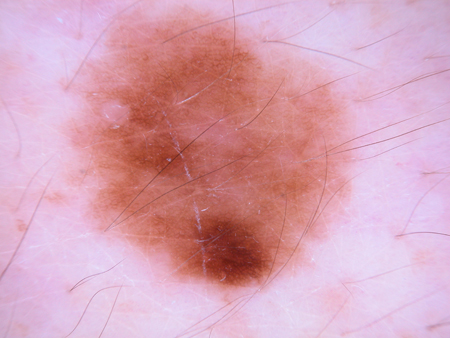 [Figure caption and citation for the preceding image starts]: Dermatoscopy of a dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: Dermatoscopy of a dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].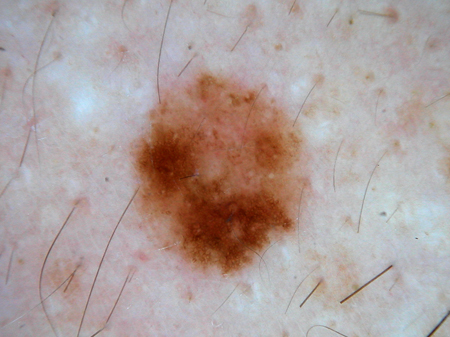 [Figure caption and citation for the preceding image starts]: Dermatoscopy of a dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
[Figure caption and citation for the preceding image starts]: Dermatoscopy of a dysplastic or Clark nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
Non-invasive imaging technologies
Several medical devices have been developed in an effort to aid primary-care physicians and dermatologists in their diagnostic accuracy of benign versus malignant pigmented melanocytic neoplasms. There are several different non-invasive imaging technologies available for research and clinical purposes. Optical generated techniques, such as optical coherence tomography (OCT) and confocal microscopy, differ from dermatoscopy or epiluminescence microscopy, which use multispectral imaging to provide visualization of the horizontal plane.[39][40] Using a hand-held device to measure the light scatter of a neoplasm, these newer multispectral imaging techniques utilize computer-generated algorithms to determine the level of disorganization, and thus malignant risk, of an individual lesion.
Spectrophotometric intracutaneous analysis (SIA) or SIAscopy was developed for lesions that are clinically atypical pigmented melanocytic neoplasms and therefore suspicious, rather than those that are clearly benign or malignant.[41][42]
SIAscopy has been advocated for use by primary-care practitioners to increase the accuracy of diagnosis of suspicious melanocytic neoplasms prior to secondary care referral. However, a randomized controlled trial in the UK found no evidence that use of SIAscopy in addition to a primary-care scoring system improved the appropriateness of specialist referral compared with systematic application of best practice (clinical history, naked eye examination, seven-point checklist) alone.[43][44]
A multi-spectral digital dermatoscope awarded US Food and Drug Administration approval is available for use specifically by dermatologists to provide a 3-dimensional morphologic picture of clinically atypical pigmented neoplasms with at least one characteristic of melanoma. In feasibility studies, depending on the parameters utilized, this device reported a 95% sensitivity and 68% specificity in the detection of melanomas.[39][45]
Despite the potential utility of such devices, the gold standard of diagnosis remains histologic examination of the neoplasm in question.
Total body photography
Some centers specializing in pigmented lesions utilize total body photography as an adjunct to help identify changes in nevi in an effort to detect melanomas at an earlier stage.[32] In its ideal execution, this practice is reassuring to patient and physician, and may result in fewer benign nevi being excised. Limitations in this approach are that changes in nevi do not necessarily imply malignant transformation, as each nevus has a natural history over the patient's life.
Biopsy
Reasons to biopsy on medical grounds for histopathologic diagnosis should be considered if melanoma is suspected clinically; if there is a history of change in the lesion, supported by physical exam; and/or if there is a high suspicion of atypical features suggestive of melanoma.[46]
Dysplastic nevi are one kind of benign nevus that can be clinically difficult to distinguish from melanoma, as they possess some features of the ABCDE criteria but to a lesser degree than melanomas. It is important to understand that they are a type of benign nevus and should not be indiscriminately biopsied.[17][32][47][48] Most melanomas arise de novo rather than in association with a nevus, thus prophylactic removal of nevi is not warranted.[32][47][48] Biopsy should be reserved for lesions suggestive of malignant changes; removal of all dysplastic nevi is not recommended.[1][4][5][30][31][32][47][48] If the clinician is uncertain about the distinction between melanocytic nevus and melanoma, a dermatology referral should be considered. Other reasons to biopsy or remove may be based on the patient's desires, such as if the lesion is repetitively traumatized or irritated, or bleeds or itches, and for cosmesis. Some physicians, largely plastic and reconstructive surgeons, recommend prophylactic surgical resection of giant congenital melanocytic nevi in certain cases, to decrease melanocyte load and to potentially decrease the risk of melanoma.[49] This is a controversial assertion, as one half of melanomas that arise in giant congenital nevi do so in extracutaneous sites. However, surgical excision of giant congenital nevi may be attempted for other reasons, including psychological benefit secondary to the esthetic appearance of the lesion.[49]
Biopsy technique is an important component in making the diagnosis.[5][24][46] Common methods to sample melanocytic nevi include shave, punch, and excisional biopsies. Incompletely excised nevi result in a persistent or recurrent nevus, which may show a pseudomelanoma pattern clinically and histologically. This may pose a diagnostic dilemma for the histopathologist.[50][51] An excisional biopsy, in which the entire lesion is removed with small margins, is generally the preferred method for removal of melanocytic nevi, in addition to being the most appropriate way to biopsy a pigmented lesion with clinical suspicion of melanoma.[24][32][46] Excisional biopsies remove sampling error, providing the pathologist with the entire architecture of the lesion to ensure accurate diagnosis, and are the best way to ensure complete removal of the lesion with margins of safety.[46]
Histopathologic analysis reveals a well-nested, localized collection of melanocytes with a benign architectural and cytologic appearance. Depending on the location of the melanocytes, the nevus may be classified as junctional (epidermal), dermal (in the dermis), or compound (both epidermal and dermal), and most acquired nevi are classified in this manner.
Congenital nevi involve the lower dermis, are splayed between collagen bundles, follow appendageal structures of the skin, and in giant lesions may extend into the subcutaneous fat and even involve fascia and muscle.[52]
Nevus spilus has a background of lentiginous melanocytic hyperplasia punctuated by either nested or increased solitary melanocytes.
Common blue nevi have melanin-laden spindled melanocytes in the dermis.
Halo nevi show a dense, bandlike lymphohistiocytic infiltrate in the papillary dermis, with nested melanocytes in the epidermis.
Spitz nevi have large nests of spindled or epithelioid melanocytes. Histologically they can mimic melanoma, although they are a benign process.
Atypical, dysplastic, or Clark nevi have a characteristic architecture of melanocytes situated at the tips of elongated rete-ridges that are surrounded by a thin rim of sclerosis (concentric and lamellar fibrosis).[7][17][31][47][48] Patchy lymphocytic infiltrate and melanin-containing histiocytes are also usually present in the dermis. When the intradermal component is present, the melanocytes are present within the expanded papillary dermis in the center of the lesion. The epidermal architecture found in dysplastic melanocytic nevi is not unique, and can be found in other melanocytic nevi, especially in small congenital nevi. Those who believe that dysplastic nevi are precursor lesions to melanoma practice melanocytic dysplasia, and use a histologic grading system of mild, moderate, and severe to describe the architectural disorder and cytologic atypia, implying a stepwise progression of nevus to melanoma. Presence of cytologic atypia must be "random". There is no standard approach or guidance on which grades require further excision, leaving it to the judgment of the dermatopathologist or clinician. Excision is generally not performed on mildly dysplastic nevi, but moderately or severely dysplastic nevi are routinely excised. When excision outcomes of nevi that were categorized histologically as mildly or moderately dysplastic were examined, no melanomas were detected, and a clinically significant change in diagnosis occurred very rarely, indicating that excision subsequent to biopsy-diagnosed mildly or moderately dysplastic nevi may often be unnecessary.[53][54][55][56] In light of these findings, a consensus statement was published that discourages re-excision of mild to moderately dysplastic nevi.[57] Furthermore, the WHO recommends a simpler histologic grading of low grade and high grade, abandoning the mild, moderate, and severe scale of grading.[8] The implication is that only high grade should be excised completely.
Despite the name, clinical and histologic correlation of atypia in dysplastic nevi is poor. For this reason, and because many practitioners believe that these nevi are not precursor lesions to melanoma, an alternative view is to categorize these nevi as Clark nevi. This eponym is used to emphasize that these are benign nevi with a particular histologic pattern that can be clinically difficult to distinguish from melanoma based on its fulfillment of the ABCDE criteria.
While the concordance rate among pathologists is excellent in the diagnosis of routine nevi and thick invasive melanomas, the rate is poor to fair at best for Spitz nevi, grading of dysplastic nevi, melanoma in-situ, and thin melanomas, illustrating the highly subjective nature and limitation of the diagnostic gold standard.[58]
A non-invasive gene-profiling tape stripping test has been developed with a reported sensitivity of 95% and specificity of 60%, boasting a negative predictive value of 99%.[59] As a relatively new test that has not been independently validated, it is not widely utilized as of yet. The test detects the expression of melanoma-associated genes PRAME and LINC00518 and the TERT mutation found in most melanomas.
Molecular adjunctive tests have been developed to improve the classification of melanocytic lesions. A 23-gene expression profiling assay has been developed that differentiates melanoma from nevi with a sensitivity of 93.8% and specificity of 96.2%.[60]
For histologically ambiguous spitzoid melanocytic lesions, histopathologists are increasingly relying on molecular cytogenetic analysis to facilitate diagnosis, particularly for atypical spitzoid lesions.[61] Molecular cytogenetic analysis is not recommended for diagnosis if histopathology is definitive for melanocytic nevus; molecular analysis is an adjunct test to be considered if diagnosis cannot be made equivocally with histopathology.[62] Melanomas are known to harbor chromosomal aberrations that are different from Spitz nevi and other melanocytic nevi. Aberrations in DNA copy number can be detected using fluorescent in-situ hybridization (FISH) and comparative genomic hybridization (CGH).[63] While CGH examines the entire genome, FISH detects specific loci of chromosomes for which the probes are available. Commercially available FISH probes include targets for 6p25 (RREB1), 6q23 (MYB), 11q13 (CCND1), CEP6 (centromeric probe for chromosome 6), and 9p21 (CDKN2A). Gains in 6p25 and 11q13 and homozygous deletions in 9p21 have been reported as being highly associated with melanoma.[61] The sensitivity and specificity of using FISH in the diagnosis of melanoma have been reported as reaching up to 94% and 98%, respectively.[61] While these results have been validated for straightforward lesions, validation for ambiguous melanocytic lesions is ongoing. [Figure caption and citation for the preceding image starts]: A persistent or recurrent nevusFrom the collection of Jason Lee, Thomas Jefferson University [Citation ends].
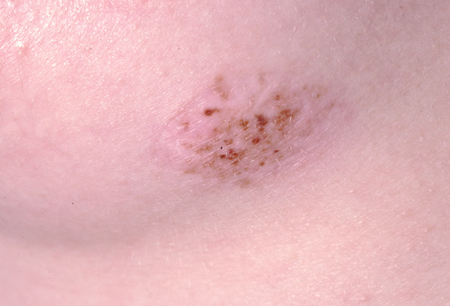
Application of PRAME (PReferentially expressed Antigen in MElanoma) immunohistochemical stain on ambiguous melanocytic lesions has been widely adopted by pathologists as most melanocytic nevi do not stain for this marker.[64]
As molecular diagnostics continue to advance, additional diagnostic tools are emerging to assist in management and diagnosis of melanocytic lesions. Mutation in the TERT promoter gene is found in approximately 80% of melanomas. The presence of mutation in the TERT gene is helpful in differentiating melanoma from a nevus.[65] If there are existing genetic test results, do not order a duplicate test unless there is uncertainty about the existing result, e.g., the result is inconsistent with the patient’s clinical presentation or the test methodology has changed.[66]
Demonstration of injection techniques used to administer local anesthetic, for allergy skin testing, and for tuberculin skin testing.
Use of this content is subject to our disclaimer