Etiology
A genetic predisposition for the formation of nevi is likely, and acquired nevi are more common in people with fair skin (skin type I) and light eyes.[1][5][18][19] Environmental factors such as sun exposure may play a role, as acquired nevi are associated with the tendency of the skin to burn and a history of severe burns; there is a possible increase in prevalence with decreasing latitude.[7][18][19][20][21]
Pathophysiology
Melanocytes are neural crest-derived cells that migrate to the epidermis during embryogenesis.[5][22] In addition to the epidermis, melanocytes are normally found in hair follicles, the uveal tract of the eye, the leptomeninges, and the cochlea. Initially found throughout the dermis after migration, they undergo presumed apoptosis in all but the head, neck, dorsal distal extremities, and presacral area. These anatomic areas coincide with the most common locations of blue nevi, which are dermal collections of melanocytes. Congenital nevi also possess intradermal melanocytes, but tend to be more infiltrative of the lower dermis, subcutis, and even deeper structures, and characteristically are in close approximation with skin appendages such as hair follicles and sweat glands. In acquired melanocytic nevi, an unknown signal presumably triggers melanocyte proliferation. Junctional nevi result from nested proliferations of these melanocytes in the epidermis. Some nevus cells are thought to migrate to the dermis from the epidermis, resulting in a compound nevus. When the epidermal component is no longer present, the nevus is termed an intradermal nevus. A halo nevus is thought to be caused by an autoimmune inflammatory response to an unknown antigen in the nevus, usually resulting in its disappearance.
Melanin, the pigment formed by melanocytes, is thought to have an ultraviolet-protective function, although some have hypothesized that the primary function of melanocytes lies in their role in antimicrobial defense and immunomodulation.[23][24]
Classification
Congenital or acquired lesions
The term "melanocytic nevi" encompasses a group of benign melanocyte-containing proliferations. There is no universally accepted coherent classification scheme of melanocytic nevi.[2][3][4] Current nomenclature is not based on uniform criteria, such as timing of onset, color, eponym, morphology, and histopathology.[2][3][4]
Broadly speaking, melanocytic nevi can be divided into congenital or acquired lesions.
Congenital melanocytic nevi
These are defined by their presence at birth; however, confusion arises when melanocytic nevi with clinical and histologic features of congenital nevi appear after 1 month of age, up to the age of 2 years.[5][6] These late-onset congenital nevi are referred to as "nevus tardive." Congenital nevi are often classified based on size, and have a histologic pattern that tends to follow adnexal structures in the skin. To add to the confusion, some acquired nevi have a congenital pattern histologically.
Acquired melanocytic nevi
These are themselves a heterogeneous group of nevi that do not fit into other defined entities. They begin to appear after 6 months of life, increase in number from childhood, and peak during the third decade.[7]
Histopathologically, both acquired and congenital nevi are further subdivided into junctional, dermal, and compound nevi depending on the location of the melanocytes: epidermal, dermal, or both, respectively.[2] Below is a common classification scheme of some of the most commonly encountered variants of melanocytic nevi.
Congenital melanocytic nevus
Small: <1.5 cm in diameter
Medium: 1.5 to 19.9 cm in diameter
Large (or giant): ≥20 cm in diameter in adults (9 cm on the scalp or 6 cm on the trunk in newborns)
Common acquired melanocytic nevus
Nevus spilus or speckled lentiginous nevus
Blue nevus
Halo nevus
Spitz nevus
Atypical, dysplastic, or Clark nevus
For those ambiguous lesions that cannot be assigned a nevus, melanoma, or intermediate category, the most recent WHO classification of melanocytic neoplasms recommends the adoption of designations IAMPUS (intraepidermal atypical melanocytic proliferation of uncertain significance), SAMPUS (superficial atypical melanocytic proliferation of uncertain significance), and MELTUMP (melanocytic tumor of uncertain malignant potential).[8] These categories have not been widely adopted. The historical poor concordance among pathologists in the diagnosis of ambiguous melanocytic lesions predicts a poor agreement of the categories. One pathologist's IAMPUS may be a nevus or melanoma in situ depending on the threshold of the pathologist and the local culture.
Advances in molecular diagnostics are changing the traditional histopathologic classification of melanocytic neoplasms to be molecular-based. The table "Currently known gene mutations and gene fusions associated with nevi" lists the currently known gene mutations and gene fusions associated with nevi.[8] The majority of commonly acquired nevi and Clark/dysplastic nevi are associated with an activating mutation in oncogene BRAF and, less frequently, NRAS.[9] Most Spitz nevi are associated with mutually exclusive kinase gene fusion while a subset of Spitz nevi, approximately 20%, harbor an activating mutation in oncogene HRAS.[10] Although melanocytic nevi and melanomas may harbor the same gene mutations, nevi are usually associated with a mutation in one oncogene and low tumor mutational burden while melanomas are associated with the acquisition of additional driver mutations and high tumor mutational burden.[11]
[Figure caption and citation for the preceding image starts]: Currently known gene mutations and gene fusions associated with neviDerived by Dr JB Lee from: Elder DE et al. WHO Classification of Skin Tumours. 4th ed. International Agency for Research on Cancer; 2018. [Citation ends].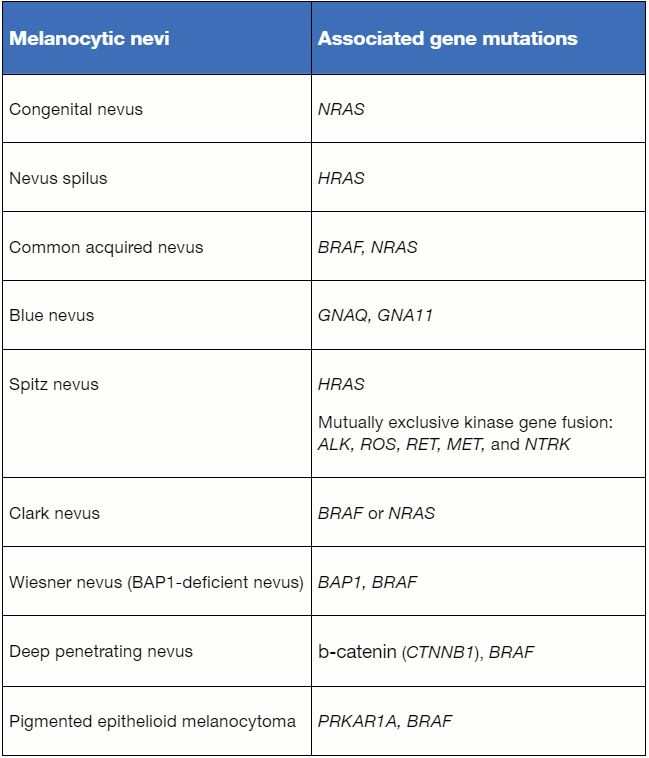
Use of this content is subject to our disclaimer