Etiology
Vitamin B12 is an essential vitamin obtained only from diet or by supplementation. Dietary sources include animal and dairy products such as meat, poultry, milk, and eggs. Stores of vitamin B12 in the liver remain in the body for years, so vitamin B12 deficiency depends on chronic, long-term deficiency.
Anything that decreases the intake or the absorption of vitamin B12 places people at risk of vitamin B12 deficiency. In general, etiologies of vitamin B12 deficiency can be categorized into:
Decreased dietary intake
Diminished gastric breakdown of vitamin B12 from food
Malabsorption from the gastrointestinal tract.
Patients at high risk of vitamin B12 deficiency include:
Vegans and strict vegetarians
History of gastric or intestinal surgery
History of atrophic gastritis
Pernicious anemia, in which autoimmune destruction of the parietal cells (which produce intrinsic factor) leads to reduced vitamin B12 absorption from the gastrointestinal tract
Gastric malabsorption.
Medications that diminish the breakdown of vitamin B12 from food sources (e.g., H2 receptor antagonists and proton-pump inhibitors) or decrease absorption of vitamin B12 (metformin) can also cause deficiency.[31][32]
Any malabsorption syndrome can place the patient at risk for vitamin B12 deficiency, such as:
Crohn disease
Celiac disease
Bacterial overgrowth syndromes.
Studies suggest a link between Helicobacter pylori infection and vitamin B12 deficiency.[33][34] However, it is unclear whether the organism, or associated atrophic gastritis, causes vitamin B12 deficiency.[35] There does not appear to be an association between H pylori infection and vitamin B12 deficiency in pregnant women.[36]
Anticonvulsant drugs (e.g., carbamazepine) have been associated with vitamin B12 deficiency.[37][38]The exact mechanism is unclear but might include interference with absorption, plasma binding, cellular metabolism, and renal excretion.
Recreational nitrous oxide (N₂O) misuse, which is increasingly prevalent, has also been linked to vitamin B12 deficiency.[39] In one global systematic review and meta-analysis, up to 85% of reported recreational users were possibly or probably vitamin B12-deficient.[40] N₂O converts the active monovalent form of vitamin B12 to its inactive bivalent form. The neurologic sequelae of N₂O-induced vitamin B12 deficiency can include neuropathy and paralysis.[41]
Pathophysiology
Dietary sources of vitamin B12 (meat, poultry, dairy) are ingested and released from food by peptic acid. Free vitamin B12 then binds to intrinsic factor (IF), which is secreted from the parietal cells of the gastric fundus. The vitamin B12-IF complex travels to the small intestine, where endocytosis occurs in the terminal ileum, by which process it is bound to transcobalamin. The transcobalamin-vitamin B12 complex (holotranscobalamin) is then released into the serum for cell utilization. Any interference in this process can place the patient at risk for vitamin B12 deficiency.[Figure caption and citation for the preceding image starts]: Absorption of vitamin B12. Dietary B12 is released from food in the stomach and binds to R-protein (haptocorrin, this complex is stable in the gastric acidic environment). The R-B12 complex then travels to the duodenum, where B12 binds to intrinsic factor (IF, secreted by gastric parietal cells and therefore lost in pernicious anemia). This B12-IF complex is carried down the small intestine until the terminal ileum, where it attaches to IF receptors and is absorbed into the bloodstream bound to transcobalamin. Over 95% of dietary B12 is absorbed through the IF pathwaySukumar N et al. Investigating vitamin B12 deficiency. BMJ. 2019 May 10;365:l1865; used with permission [Citation ends].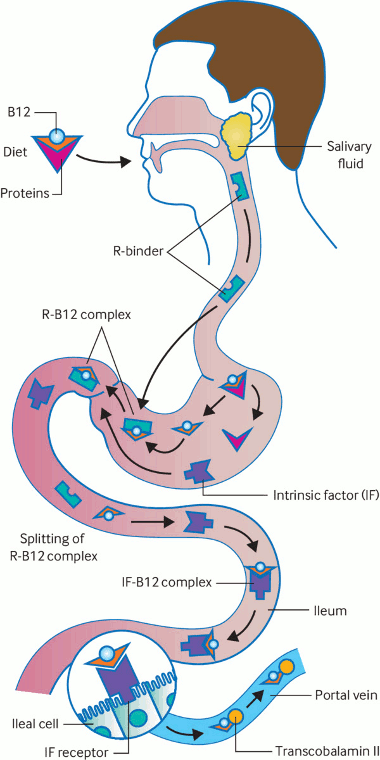
Vitamin B12 is an essential cofactor in DNA synthesis and is closely related to folate metabolism. Specifically, vitamin B12 is an important cofactor in two biochemical processes involving methylmalonic acid and homocysteine as precursors. Deficiency of vitamin B12 impairs the conversion of methylmalonic acid to succinyl co-A. Deficiency of vitamin B12 or folate impairs the conversion of homocysteine to methionine. Methionine is critical in the production of S-adenosylmethionine, which is thought to be important in neural function. Vitamin B12 and folate are thought to be integral in normal hematopoiesis and bone marrow function.
Prolonged and severe deficiency in vitamin B12 can cause neurologic and hematologic disorders, and may manifest with psychiatric symptoms.[42][43]
Use of this content is subject to our disclaimer