Approach
The diagnosis of thoracolumbar fractures is primarily based on the mechanism of injury and clinical presentation. Clinical exam has a low to very low sensitivity for identifying thoracolumbar spine injuries.[11][13] The use of appropriate imaging is needed for further evaluation and planning of treatment, whether non-operative or operative.[11] Caution should be exercised to avoid delayed diagnosis of thoracolumbar fractures in multiple injury patients. The incidence of misdiagnosis of thoracolumbar fractures is as high as 19%.[44]
In a patient with impaired consciousness, observations from friends, family members, ambulance crew, nurses or doctors at the referring hospital, or other people who have been in contact with the patient are required.
It is important to obtain a good history to find out the mechanism of injury (e.g., fall from a height, road traffic crash, sport injury), which may indicate the severity of injury.[10] The examination findings to look for include step deformity and pain. The absence of spinal pain or deformity, including the absence of neurologic deficit, may suggest that there is no thoracolumbar fracture. However, if such pain or deformity is present, then the occurrence of fractures or other injuries (e.g., ligamentous injury, hematomas, paravertebral soft tissue injuries) can be identified only by further imaging (magnetic resonance imaging [MRI], computed tomography [CT]).
Initial assessment and resuscitation
Initial evaluation at the scene includes:[10][11][45][46]
Airway maintenance with spinal motion restriction (SMR). When SMR is indicated in adults, it should be applied to the entire spine due to the risk of noncontiguous injuries. A critical component of SMR is the application of an appropriately sized cervical collar.[11] The head, neck, and torso should be kept in alignment by placing the patient on a long backboard, a scoop stretcher, a vacuum mattress, or an ambulance cot.[11][47] The term “SMR” is recommended instead of “immobilization,” because current techniques limit or reduce undesired motion of the spine, but they do not provide true spinal immobilization.[11][47]
Breathing and ventilation (assess respiratory rate, depth of breathing, and oxygen saturation; shallow breathing with tachypnea may suggest higher cervical fracture).
Circulation with hemorrhage control (assess heart rate, blood pressure, and capillary refill time; use a hypotensive resuscitation strategy to maintain a target mean arterial blood pressure of 50 to 65 mmHg if there is ongoing bleeding).[48][49] The Congress of Neurological Surgeons (CNS) and the American Association of Neurological Surgeons (AANS) state there is insufficient evidence to recommend for or against the use of active maintenance of arterial blood pressure after thoracolumbar spinal cord injury. However, in light of published data from pooled (cervical and thoracolumbar) spinal cord injury patient populations, the CNS/AANS suggest clinicians may choose to maintain mean arterial blood pressures >85 mmHg to improve neurologic outcomes.[50]
Disability (assess Glasgow Coma Scale [GCS] score, pupil size and reaction bilaterally, and lateralizing signs).
Exposing the patient to inspect for any obvious site of major injury.
If acute spinal cord injury is suspected (with or without vertebral column injury) the patient should be transferred to the nearest trauma center so that life-threatening conditions can be identified and treated before transfer to a spinal cord injury center.[46] In the acute phase following spinal cord injury, intravenous morphine is first line for pain relief. Intravenous ketamine is a second-line agent; intranasal ketamine can be used if there is a delay in getting intravenous access.[46]
History
A careful history focusing on the spinal pain, paralysis, or sensory disturbance is required.[11] It is very important to find out the timing of the events: for example, if the patient reports any movement or sensation of the affected parts of the body before paralysis or full numbness. However, absence of such symptoms with a distracting injury may be misleading.[11] In the case of pain, the level of back pain reported by the patient usually does not correlate with the level of injury within the segments of the spinal cord due to discordant growth rate between the spinal (bony) column and the spinal cord.[11]
According to the American College of Surgeons, significant findings during assessment for thoracic or lumbosacral spine injury that necessitate in-line SMR include:[11]
Acutely altered level of consciousness (e.g., GCS <15, evidence of intoxication)
Midline neck or back pain and/or tenderness
Focal neurologic signs and/or symptoms (e.g., numbness or motor weakness)
Anatomic deformity of the spine
Distracting circumstances or injury (e.g., long bone fracture, degloving, or crush injuries, large burns, emotional distress, communication barrier, etc.) or any similar injury that impairs the patient’s ability to contribute to a reliable exam.
If trivial trauma is associated with lower-energy injury, then osteoporosis or other sinister causes (e.g., neoplasia, metabolic disorders) need to be ruled out.[11] Additionally, past medical history may reveal risk factors including concomitant osteoporosis, previous vertebral fracture, underlying neoplasia (e.g., multiple myeloma, bony metastasis), or an underlying metabolic or inflammatory disorder (e.g., osteogenesis imperfecta, osteoporosis, rheumatoid arthritis, ankylosing spondylitis).[11][13][Figure caption and citation for the preceding image starts]: MRI thoracic spine: sagittal view (T2-weighted sequence) showing a pathologic fracture of the T10 vertebral body caused by multiple myelomaFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends]. [Figure caption and citation for the preceding image starts]: MRI lumbar spine: sagittal view (T2-weighted sequence) showing an osteoporotic fracture of the T12 vertebral bodyFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI lumbar spine: sagittal view (T2-weighted sequence) showing an osteoporotic fracture of the T12 vertebral bodyFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends].
Physical exam
The primary survey should focus on hemorrhage control, airway, breathing, circulation, disability, and exposure. Cervical and thoracolumbar SMR should be maintained throughout this phase, until the spine is further evaluated during the secondary survey.[11] Vital signs should be measured. A combination of hypotension and bradycardia in the setting of potential spinal cord injury may be indicative of neurogenic shock.
The level of consciousness should be formally assessed using the GCS. [ Glasgow Coma Scale Opens in new window ] Impairment of consciousness might impact the reliability of the history and physical exam findings.[11] Extremity or pelvic fractures, burns or other injuries, such as to the brachial plexus may affect the results of sensory or motor deficit evaluation.[11]
The primary goal during the “disability” evaluation, after assessing the GCS score and pupillary response, is to identify any lateralizing signs by conducting a rapid assessment of motor function and reflexes in the extremities.[11] Alert and cooperative patients can simply be asked to raise upper and lower extremities sequentially while the clinician observes for any differences.[11] Absence of equal movement in the upper and/or lower extremities with or without a gross sensory deficit may be suggestive of a spinal cord injury.[11] Loss of bowel and bladder function (urinary retention or urinary/fecal incontinence) and/or priapism in males can suggest acute spinal cord injury; differentials should be excluded.[11][51]
The secondary survey aims to obtain a full and detailed history and physical exam after completion of the primary survey. This includes a structured physical exam of the entire spine with in-line SMR and the backboard removed.[11] Ideally, complete evaluation of the thoracolumbar spine requires the patient to be in standing, sitting, supine, and prone positions. However, this is not feasible when SMR must be maintained during the acute phase of trauma care (i.e., until an injury has been ruled out). With the patient logrolled using inline SMR, the back should be inspected for obvious spinal deformities, contusions, abrasions, hematomas, and/or open wounds. The cervical spine region should also be inspected.[11] The entire thoracolumbar and sacral midline should be systematically palpated to evaluate for pain, tenderness, step offs, gaps or other deformities.[11] The physical exam of the thoracolumbar spine has very low sensitivity.[11] The level of pain and/ or tenderness often does not correlate with the level of injury on imaging.[11] However, a normal exam has a low sensitivity in ruling out spinal injury.[52][53]
Unconscious patients should be assessed for signs indicating the level of spinal cord function:
Any spontaneous movements
Response to noxious stimuli
Muscle tone: increased tone (upper motor neuron [UMN] lesion); decreased tone (lower motor neuron [LMN] lesion)
Reflexes: hyperreflexia (suggests UMN lesion); hypo- or areflexia (suggests LMN lesion)
Rectal tone: decreased (suggests LMN lesion).
Conscious patients require inspection, palpation, and full neurologic exam.
Once the neurologic assessment is completed in a conscious patient, then American Spinal Injury Association (ASIA) International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) scoring of motor and sensory components is used. ASIA: international standards for neurological classification of spinal cord injury Opens in new window
[Figure caption and citation for the preceding image starts]: ASIA Standard Neurological Classification of Spinal Cord Injury: impairment scaleAmerican Spinal Injury Association, used with permission [Citation ends].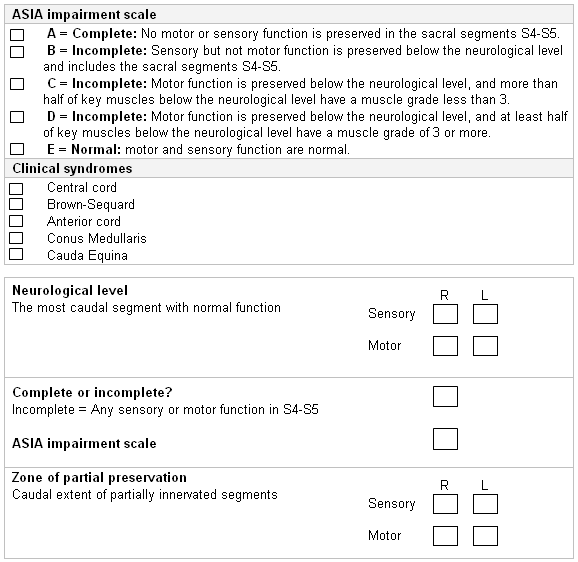
The presence of bruising should be noted. Deformity (step deformity or swelling) over the spine indicates the likely level of injury. On palpation of the spine, the level of tenderness elicited correlates with the level of spinal (bony) column injury.
Acute numbness or paresthesia may be due to compression of the spinal cord or nerve root(s). A careful clinical assessment of the level of neurologic injury, including dermatomal distribution of sensation, is necessary to enable optimal analysis of spinal imaging. The neurologic injury level is defined as the most caudal segment of the spinal cord with normal sensory function on both sides.[11] Simultaneous right/left evaluation of individual dermatome sensation is likely to improve sensitivity in sensation function assessment.
Muscle spasticity suggests an UMN lesion, while hypotonia or flaccidity suggests spinal shock (as a temporary feature) or a LMN lesion.[11] Clonus is said to be abnormal if the foot flexes involuntarily >3 beats when the foot is forcefully extended. Abnormal clonus suggests an UMN lesion.
Other signs of an UMN lesion include Hoffman sign (flicking of the nail bed of the middle finger results in sudden twitch of other fingers ipsilaterally) which is related to cervical spine cord compression, and a positive Babinski sign (on forceful stroking of the lateral or plantar aspect of the foot, there is extension of the big toe and fanning out of the other toes ipsilaterally).
Loss of anal sphincter reflex suggests a spinal cord injury, but is not specific for certain levels.
The bulbocavernosus reflex is subserved by S4-S5 nerve root and elicited by squeezing the glans penis or pulling gently on the bladder indwelling catheter. The absence of this reflex indicates that spinal shock is likely present and precludes definitive evaluation about completeness of spinal cord injury.[11] The return of the bulbocavernosus reflex heralds the end of spinal shock. Absence of this reflex may also indicate sacral spinal cord injury or injury to sacral roots.
Urinary incontinence is usually due to high-level injury causing damage to the spinal cord. The impairment of the inhibitory modulation of the descending fibers will cause the bladder to contract at small bladder volumes, resulting in incontinence (hypertonic bladder). In addition, high-level injury may lead to urinary retention with overflow incontinence in the acute period, and this will be painless (i.e., the patient has no sensation of a full bladder) if the injury is complete. Persistent, painless urinary retention suggests injury within the cauda equina affecting the nerves supplying the bladder, resulting in retention.
Multilevel spinal fractures are present in 15% to 20% of spinal injury patients, and patients with spinal cord injuries commonly have other associated injuries, such as concurrent multiple system injuries (80%) and head injuries (41%).[30][31]
If a spinal cord injury is identified, the level of injury should be assessed using the ISNCSCI, as recommended by the American College of Surgeons).[11]
Spinal shock versus neurogenic shock
Following injury to the spinal cord, there may be an immediate depolarization of the spinal nerve fibers followed by repolarization of nerve fibers.[37]
Neurogenic shock
Caused by spinal cord injuries at or above the level of T6.
Subsequent repolarization diminishes the effect of a descending sympathetic pathway, leading to loss of vasomotor tone of the blood vessels and pooling of blood, mainly in the periphery, leading to hypotension.
In addition, diminished sympathetic input to the heart leads to bradycardia.
Treatment is with fluid resuscitation. Inotrope use may be necessary.[11]
Spinal shock
Subsequent repolarization produces temporary features of an LMN lesion (flaccidity, loss of tone, diminished reflexes).
Clinically, it is suggested by:
Disruption of all cord functions below the level of injury, including reflexes (the presence of reflexes indicates absence of spinal shock).[11]
There is a lack of consensus on defining the end of spinal shock, but some theories suggest that the end of spinal shock is heralded by the return of reflexes, for example:
The appearance of the bulbocavernosus reflex, occurring within several days of injury
The return of deep tendon reflexes which can take several weeks
The return of reflexive bladder function.
Predictions regarding functional recovery are more reliable if based on neurologic exams 72 hours to 1 week after a trauma.[54]
Generally, spinal shock is not considered as a limiting factor in making decisions about operative or non-operative treatment. If the patient needs urgent spinal decompression, waiting for 72 hours for the spinal shock to subside completely may be detrimental.[Figure caption and citation for the preceding image starts]: Spinal cord injuryCreated by Dr B. Nurboja [Citation ends].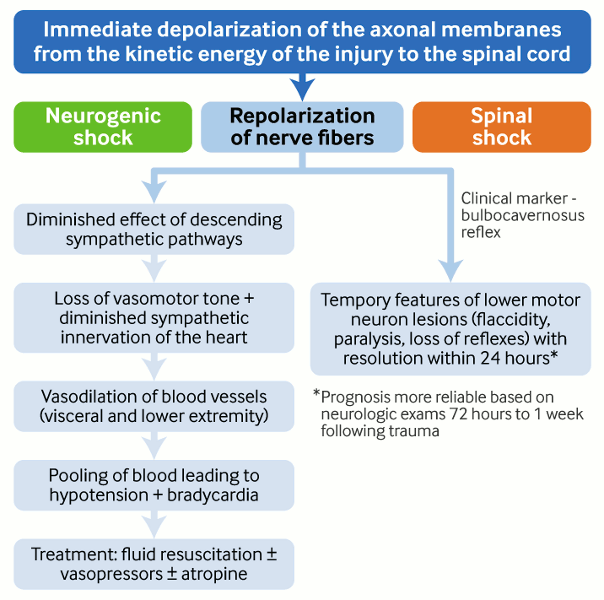
Ongoing bulbocavernosus reflex presence suggests the spinal shock is ongoing and therefore prognosis of further recovery or worsening cannot be made at this stage.[11]
Imaging in adults
After using appropriate clinical decision rules and assessing risk for spinal cord fracture or spinal cord injury during the primary and secondary surveys, patients at risk for spinal trauma should be further evaluated with dedicated imaging studies.[10][11] Imaging of the thoracolumbar spine in acute spinal trauma should be undertaken if any of the following are present: 1) back pain or midline tenderness; 2) local signs of thoracolumbar injury (e.g., bruising); 3) abnormal neurologic signs; 4) cervical spine fracture; 5) GCS score <15; 6) major distracting injury; and 7) alcohol or drug intoxication.[55]
Noncontrast, multidetector computed tomography (MDCT) is the initial imaging modality of choice to evaluate the thoracolumbar spine.[11] Slice thickness should not exceed 5 mm.[11] Thoracolumbar spine imaging may be reconstructed from images concurrently obtained for evaluation of the chest, abdomen, and pelvis in a multitrauma patient. Routine reformatting of these images is not required. A more selective imaging approach is appropriate for patients with a high suspicion of spine injury or when an injury is identified on the nonreformatted images.[11] The sensitivity of CT reaches almost 100%, and it provides details about vertebral fracture morphology (comminution), presence of bony (posterior wall) fragments in the spinal canal, and indirect signs of disruption of the posterior ligamental complex.[10] The main limitation of CT rests in its relative inability to detect changes in soft tissues, including the spinal cord and ligamentous structures.[11][56][57] Despite the high sensitivity of the MDCT in identifying bony abnormalities, interpretation may be difficult in patients with severe degenerative changes or osteopenia.[11]
Fractures found in one level of the spine indicate an increased risk of spinal fractures elsewhere. Thus, identification of a spinal fracture may imply a need to survey the remainder of the spine.[13]
Plain radiographs of the cervical and thoracolumbar spine are not recommended by the American College of Surgeons in the initial screening of spinal trauma because of their low sensitivity.[11] CT has a reported sensitivity of 94% to 100% for identifying thoracolumbar spine fractures, whereas radiographs have a reported sensitivity of 49% to 62% for identifying thoracic spine fractures and 67% to 82% sensitivity for identifying lumbar spine fractures.[10][13] Chest and abdominal x-rays taken as part of trauma assessment are not adequate to assess vertebral fractures and alignment.[11][58] If screening of the thoracolumbar spine is performed using radiographs, the American College of Radiology recommends that imaging should consist of anteroposterior and lateral radiographs of the thoracic and lumbar spine with additional “swimmer’s lateral” view of the upper thoracic spine if this region is obscured by the overlying shoulders.[13]
MRI scan of the spine is indicated if there is any neurologic deficit despite normal appearance on a CT spine as some conditions are apparent only on MRI (e.g., epidural hematoma or disk protrusions causing spinal cord compression). MRI is also indicated if there are symptoms or signs of spinal cord, conus medullaris, or nerve root injury.[13] MRI is the only modality for evaluating the internal structure of the spinal cord.[11][56][57] AOSpine guidelines suggest that when feasible and as long as there are no contraindications, MRI should be used in spinal cord injury patients prior to surgical intervention to improve clinical decision-making, and before or after surgical intervention to predict the neurologic outcome.[59]
MRI spine will identify intramedullary lesions (e.g., post-traumatic cysts, hematomas, edema) and extramedullary compressions (e.g., disk, hematoma, bone fragments), and allow assessment of a posterior ligamentous complex.[8][10][50]
Evaluation of patients with compressive vertebral fractures requires performing an MRI including the Short Tau Inversion Recovery (STIR) sequence to distinguish an already healed from an unhealed fracture.
CT myelography may be required if MRI spine is unavailable or contraindicated (e.g., in patients with metallic implants, hemodynamic instability, or severe claustrophobia).
Whole-body CT, consisting of scanogram from vertex to toes followed by CT from vertex to mid-thigh, is indicated in adults with multiple injuries with major trauma and suspected spinal column injury.[11][46][Figure caption and citation for the preceding image starts]: MRI thoracic spine: sagittal view (T2-weighted sequence) showing a pathologic fracture of the T10 vertebral body caused by multiple myelomaFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends]. [Figure caption and citation for the preceding image starts]: MRI lumbar spine: sagittal view (T2-weighted sequence) showing an osteoporotic fracture of the T12 vertebral bodyFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI lumbar spine: sagittal view (T2-weighted sequence) showing an osteoporotic fracture of the T12 vertebral bodyFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends]. [Figure caption and citation for the preceding image starts]: MRI lumbar spine: axial view (T2-weighted sequence) showing an osteoporotic fracture of the T12 vertebral bodyFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends].
[Figure caption and citation for the preceding image starts]: MRI lumbar spine: axial view (T2-weighted sequence) showing an osteoporotic fracture of the T12 vertebral bodyFrom the personal collection of Dr B. Nurboja and Mr D. Choi [Citation ends].
Imaging in children (age <16 years)
CT is indicated in children with suspected thoracolumbar injury if the initial x-ray is abnormal. MRI is preferred to CT in children where cervical injury is suspected. If a new fracture of the spinal column is identified, the rest of the spine should be imaged.[46]
Clinical judgment should be used to limit CT to the body area where further radiologic assessment is required because of the increased risk of cancer (including thyroid cancer) in children following CT scan exposure.[46][60] Therefore, avoid routine use of whole-body CT in pediatric trauma patients for this reason.[46][61]
Clinicians should be alert to the possibility of thoracolumbar fractures if physical abuse of a child is suspected. Studies of physically abused children showed that 1% of nonaccidental head injuries are associated with spinal trauma; 1% to 3% of all fractures in this group of patients are to the vertebrae.[62] In a systematic review of 19 case series of children with inflicted spinal injuries, where 23 out of the 25 children examined were under 2 years old, there were 12 thoracolumbar fractures, of which 75% were fracture-dislocations and 25% were vertebral compression fractures. The review recommended that all children with suspected nonaccidental injuries who are under 2 years of age should have thoracolumbar x-ray imaging.[63]
Classification of fractures
It is advisable to classify the fractures into one of the recognized classification systems, such as the Denis or AO classification systems.[2][3] These are both validated classification systems that are useful for the planning of non-operative or operative management. The AO classification is more commonly used, as it provides a comprehensive classification describing the nature of injury, degree of instability, and prognostic aspects that are important for choosing the most appropriate treatment.[56][Figure caption and citation for the preceding image starts]: Modified Magerl (AO/ASIF) classification of thoracolumbar injuriesContent adapted by author from Gertzbein SD. Spine update: classification of thoracic and lumbar fractures. Spine (Phila Pa 1976). 1994 Mar 1;19(5):626-8 [Citation ends].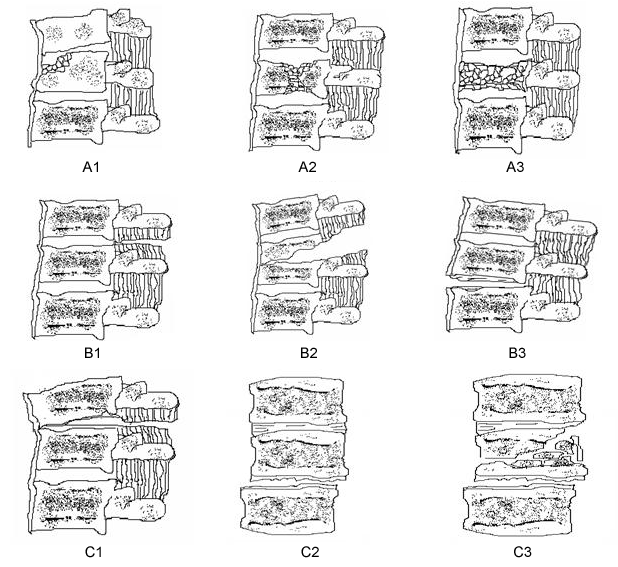
Evaluation of fracture stability
Further treatment relies on evaluation of the stability of the fracture.
Spinal instability is defined as "the loss of the ability of the spine under physiologic loads to maintain relationships between vertebrae in such a way that there is neither damage nor subsequent irritation to the spinal cord or its nerve roots, and in addition there is no development of incapacitating deformity or pain."[64]
When discussing fracture instability, there are two major concepts to remember:
Neurologic instability of the spine: defined as inability of the spine to protect the spinal cord, cauda equina, and nerve roots. An example would be ligamentous disruptions within the vertebrae, producing spinal instability severe enough to cause injury to neural structures, leading to neurologic deficit.
Mechanical instability: defined as inability to withstand physiologic demands without pain, deformity, abnormal motion, or neural compression. An example would be traumatic spondylolisthesis allowing abnormal movement of spinal segments, causing pain.
Fracture stability can be established on imaging by performing MRI of the thoracolumbar region, which may show ligamentous complex disruptions.
Evaluation of lumbar radiculopathy
Do not order needle electromyography for isolated back pain after a motor vehicle accident. If back pain is not associated with lower limb pain, lower limb tingling, lower limb weakness, or lower limb numbness, needle electromyography does not improve outcomes.[65]
Use of this content is subject to our disclaimer