Most cases of PP are found on ultrasound ordered for another reason (e.g., dating, fetal anatomic survey, undergoing IVF). Risk factors include uterine scarring (most commonly due to prior cesarean section), advanced maternal age, smoking, previous multiple pregnancies/short interpregnancy intervals or miscarriages/induced abortions, prior PP, infertility treatment, and illicit drug use.
Ultrasound is both sensitive and specific for the diagnosis of PP, although this is operator dependent.[36]Dashe JS, McIntire DD, Ramus RM, et al. Persistence of placenta previa according to gestational age at ultrasound detection. Obstet Gynecol. 2002 May;99(5 Pt 1):692-7.
http://www.ncbi.nlm.nih.gov/pubmed/11978274?tool=bestpractice.com
It may be performed by a transabdominal, transvaginal, translabial, or transperineal approach, although transabdominal ultrasound is recommended by the American College of Radiology and the American College of Obstetricians and Gynecologists as the initial procedure.[37]Shipp TD, Poder L, Feldstein VA, et al; Expert Panel on GYN and OB Imaging, American College of Radiology. ACR appropriateness criteria: second and third trimester vaginal bleeding. J Am Coll Radiol. 2020 Nov;17(11s):S497-504.
https://www.jacr.org/article/S1546-1440(20)30942-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33153560?tool=bestpractice.com
[38]American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 175: ultrasound in pregnancy. Dec 2016 [internet publication].
http://www.ncbi.nlm.nih.gov/pubmed/27875472?tool=bestpractice.com
The Society of Obstetricians and Gynaecologists of Canada, the Royal College of Obstetricians and Gynaecologists, and the Society for Maternal-Fetal Medicine meanwhile recommend transvaginal ultrasound, stating that it is more accurate for the diagnosis of PP than a transabdominal approach, with a sensitivity of 88% and a specificity of 99%.[1]Jain V, Bos H, Bujold E; Society of Obstetricians and Gynaecologists of Canada. Guideline no. 402: diagnosis and management of placenta previa. J Obstet Gynaecol Can. 2020 Jul;42(7):906-17.e1.
http://www.ncbi.nlm.nih.gov/pubmed/32591150?tool=bestpractice.com
[2]Royal College of Obstetricians and Gynaecologists. Placenta praevia and placenta accreta: diagnosis and management. Green-top guideline no. 27a. Sep 2018 [internet publication].
https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg27a
[5]Society for Maternal-Fetal Medicine. SMFM consult series #44: management of bleeding in the late preterm period. Oct 2017 [internet publication].
https://www.smfm.org/publications/249-smfm-consult-series-44-management-of-bleeding-in-the-late-preterm-period
[Figure caption and citation for the preceding image starts]: Placenta previa (previously known as complete previa) at 22 weeksFrom the teaching collection of Janet R. Albers, MD [Citation ends].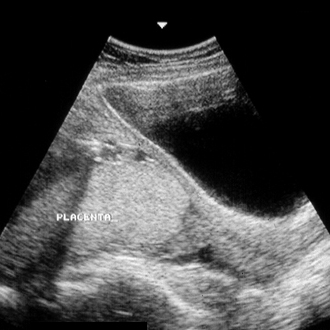 [Figure caption and citation for the preceding image starts]: Placenta previa (previously known as complete previa) at 32 weeksFrom the teaching collection of Janet R. Albers, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Placenta previa (previously known as complete previa) at 32 weeksFrom the teaching collection of Janet R. Albers, MD [Citation ends].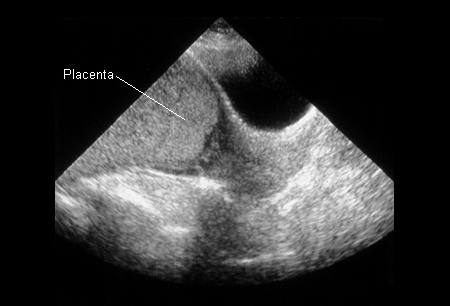
Women with previous cesarean section (or other uterine scarring)
All women with a history of cesarean section should undergo ultrasound to look for placental location.[3]Reddy UM, Abuhamad AZ, Levine D, et al. Fetal imaging: executive summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. J Ultrasound Med. 2014 May;33(5):745-57.
http://www.ncbi.nlm.nih.gov/pubmed/24764329?tool=bestpractice.com
If PP is suspected, referral should be made for color flow Doppler ultrasound to screen for placenta accreta spectrum (in which the placenta is adherent to the underlying myometrium).[2]Royal College of Obstetricians and Gynaecologists. Placenta praevia and placenta accreta: diagnosis and management. Green-top guideline no. 27a. Sep 2018 [internet publication].
https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg27a
[3]Reddy UM, Abuhamad AZ, Levine D, et al. Fetal imaging: executive summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. J Ultrasound Med. 2014 May;33(5):745-57.
http://www.ncbi.nlm.nih.gov/pubmed/24764329?tool=bestpractice.com
[4]American College of Obstetricians and Gynecologists / Society for Maternal-Fetal Medicine. ACOG SMFM obstetric care consensus #7: placenta accreta spectrum. Dec 2018 [internet publication].
https://www.acog.org/clinical/clinical-guidance/obstetric-care-consensus/articles/2018/12/placenta-accreta-spectrum
If placenta accreta spectrum cannot be reliably excluded on ultrasound, MRI of the placenta should be obtained.[2]Royal College of Obstetricians and Gynaecologists. Placenta praevia and placenta accreta: diagnosis and management. Green-top guideline no. 27a. Sep 2018 [internet publication].
https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg27a
[4]American College of Obstetricians and Gynecologists / Society for Maternal-Fetal Medicine. ACOG SMFM obstetric care consensus #7: placenta accreta spectrum. Dec 2018 [internet publication].
https://www.acog.org/clinical/clinical-guidance/obstetric-care-consensus/articles/2018/12/placenta-accreta-spectrum
[15]Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006 Apr;107(4):927-41.
http://www.ncbi.nlm.nih.gov/pubmed/16582134?tool=bestpractice.com
[39]Neilson JP. Interventions for suspected placenta praevia. Cochrane Database Syst Rev. 2003;(2):CD001998.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001998/full
http://www.ncbi.nlm.nih.gov/pubmed/12804418?tool=bestpractice.com
[40]Vladareanu R, Onofriescu M, Mihailescu D, et al. Magnetic resonance imaging in obstetrics. Rev Med Chir Soc Med Nat Iasi. 2006 Jul-Sep;110(3):567-74.
http://www.ncbi.nlm.nih.gov/pubmed/17571547?tool=bestpractice.com
[41]Abramowicz JS, Sheiner E. In utero imaging of the placenta: importance for diseases of pregnancy. Placenta. 2007 Apr;28(suppl A):S14-22.
http://www.ncbi.nlm.nih.gov/pubmed/17383721?tool=bestpractice.com
[42]Palacios Jaraquemada JM, Bruno CH. Magnetic resonance imaging in 300 cases of placenta accreta: surgical correlation of new findings. Acta Obstet Gynecol Scand. 2005 Aug;84(8):716-24.
https://obgyn.onlinelibrary.wiley.com/doi/10.1111/j.0001-6349.2005.00832.x
http://www.ncbi.nlm.nih.gov/pubmed/16026395?tool=bestpractice.com
Asymptomatic women
If PP or a low-lying placenta are detected at the routine anatomic scan (typically 18 to 22 weeks' gestation), a repeat ultrasound should be performed at approximately 32 weeks to reassess placental location.[1]Jain V, Bos H, Bujold E; Society of Obstetricians and Gynaecologists of Canada. Guideline no. 402: diagnosis and management of placenta previa. J Obstet Gynaecol Can. 2020 Jul;42(7):906-17.e1.
http://www.ncbi.nlm.nih.gov/pubmed/32591150?tool=bestpractice.com
[2]Royal College of Obstetricians and Gynaecologists. Placenta praevia and placenta accreta: diagnosis and management. Green-top guideline no. 27a. Sep 2018 [internet publication].
https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg27a
[38]American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 175: ultrasound in pregnancy. Dec 2016 [internet publication].
http://www.ncbi.nlm.nih.gov/pubmed/27875472?tool=bestpractice.com
In women with a persistent low-lying placenta or PP at 32 weeks, a further ultrasound should be arranged at 36 weeks to aid delivery planning.[1]Jain V, Bos H, Bujold E; Society of Obstetricians and Gynaecologists of Canada. Guideline no. 402: diagnosis and management of placenta previa. J Obstet Gynaecol Can. 2020 Jul;42(7):906-17.e1.
http://www.ncbi.nlm.nih.gov/pubmed/32591150?tool=bestpractice.com
[2]Royal College of Obstetricians and Gynaecologists. Placenta praevia and placenta accreta: diagnosis and management. Green-top guideline no. 27a. Sep 2018 [internet publication].
https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg27a
Women presenting with painless bleeding in late second or third trimester
If a woman presents with second- or third-trimester bleeding and PP has not already been diagnosed, a history should be taken to include known risk factors for PP. These include advanced maternal age, uterine scarring (most commonly due to prior cesarean section), prior PP, and infertility treatments. Other risk factors include previous miscarriage or induced abortion, multiparity/short interpregnancy intervals, and smoking or illicit drug use. With the exception of uterine scarring, these risk factors are neither sensitive nor specific.
Very careful speculum examination may be used to exclude cervical or vaginal hemorrhage as a cause of bleeding. In women in early labor with mild bleeding, this should be done under "double setup" to allow conversion to an immediate cesarean section in case of massive vaginal bleeding. Digital vaginal examination should never be performed before PP is ruled out by other means.[43]Chilaka VN, Konje JC, Clarke S, et al. Practice observed: is speculum examination on admission a necessary procedure in the management of all cases of antepartum haemorrhage? J Obstet Gynaecol. 2000 Jul;20(4):396-8.
http://www.ncbi.nlm.nih.gov/pubmed/15512595?tool=bestpractice.com
If time permits (e.g., where there is no evidence of fetal compromise or excessive bleeding), urgent ultrasound should be obtained.[37]Shipp TD, Poder L, Feldstein VA, et al; Expert Panel on GYN and OB Imaging, American College of Radiology. ACR appropriateness criteria: second and third trimester vaginal bleeding. J Am Coll Radiol. 2020 Nov;17(11s):S497-504.
https://www.jacr.org/article/S1546-1440(20)30942-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33153560?tool=bestpractice.com
Urgent consultation with suitably experienced physicians able to perform a cesarean section and manage attendant complications (e.g., cesarean hysterectomy) is recommended.[44]Sakornbut E, Leeman L, Fontaine P. Late pregnancy bleeding. Am Fam Physician. 2007 Apr 15;75(8):1199-206.
https://www.aafp.org/afp/2007/0415/p1199.html
http://www.ncbi.nlm.nih.gov/pubmed/17477103?tool=bestpractice.com
[45]Magann EF, Cummings JE, Niederhauser A, et al. Antepartum bleeding of unknown origin in the second half of pregnancy: a review. Obstet Gynecol Surv. 2005 Nov;60(11):741-5.
http://www.ncbi.nlm.nih.gov/pubmed/16250922?tool=bestpractice.com
In assessing the hemodynamic effects of bleeding, it is important to remember that most young, otherwise healthy pregnant women tend to have slightly low blood pressure and to be slightly tachycardic. Hemoglobin normally decreases in pregnancy. The value varies with gestational age. It may be as low as 10 mg/dL in mid-pregnancy. Iron deficiency (and, in certain genetic groups, thalassemia) commonly coexists with pregnancy and may result in an even lower hemoglobin.
Type and screen and crossmatch for at least 4 units of packed red blood cells (and inform transfusion service of possibility of the need for massive transfusion). Follow with serial complete blood counts (frequency depends on degree of bleeding). Consider assessing international normalized ratio (INR)/partial thromboplastin time (PTT), fibrinogen, and fibrinogen degradation products if there is any evidence of disseminated intravascular coagulation, such as petechiae, ecchymosis, gangrene, mental disorientation, hypoxia, hypotension, or gastrointestinal bleeding.
A fetomaternal hemorrhage involving a rhesus (Rh)-positive fetus to an Rh-negative mother has significant implications for fetal morbidity in subsequent pregnancies. Women who are Rh negative should have a Kleihauer-Betke test to help determine the degree of hemorrhage, and the need for and amount of Rho(D) immune globulin that is required for prophylaxis for Rh disease in subsequent pregnancies.[5]Society for Maternal-Fetal Medicine. SMFM consult series #44: management of bleeding in the late preterm period. Oct 2017 [internet publication].
https://www.smfm.org/publications/249-smfm-consult-series-44-management-of-bleeding-in-the-late-preterm-period
[46]American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017 Aug;130(2):e57-70.
https://journals.lww.com/greenjournal/Fulltext/2017/08000/Practice_Bulletin_No__181__Prevention_of_Rh_D.54.aspx
http://www.ncbi.nlm.nih.gov/pubmed/28742673?tool=bestpractice.com
Alpha-fetoprotein (AFP) testing is usually offered routinely as part of triple or quadruple testing to screen for neural tube defects and other congenital abnormalities. If a second- or third-trimester PP is diagnosed in a woman with an abnormal AFP level, the index of suspicion for invasive placentation should be high and an ultrasound and/or magnetic resonance imaging scan considered.[47]Gagnon A, Wilson RD, Audibert F, et al; Society of Obstetricians and Gynaecologists of Canada Genetics Committee. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008 Oct;30(10):918-32.
http://www.ncbi.nlm.nih.gov/pubmed/19038077?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: Placenta previa (previously known as complete previa) at 32 weeksFrom the teaching collection of Janet R. Albers, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Placenta previa (previously known as complete previa) at 32 weeksFrom the teaching collection of Janet R. Albers, MD [Citation ends].