Pouch or rectal screening for patients with FAP/attenuated FAP following colectomy[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2.
https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7
http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
[44]Ozdemir Y, Kalady MF, Aytac E, et al. Anal transitional zone neoplasia in patients with familial adenomatous polyposis after restorative proctocolectomy and IPAA: incidence, management, and oncologic and functional outcomes. Dis Colon Rectum. 2013;56:808-814.
http://www.ncbi.nlm.nih.gov/pubmed/23739186?tool=bestpractice.com
Pouchoscopy should be performed annually after ileal pouch-anal anastomosis.
Following ileorectal anastomosis, proctoscopy should be performed every 6-12 months.
If the patient has had an ileostomy, careful visualization and stoma inspection by ileoscopy to evaluate for polyps or malignancy should be carried out annually.
Gastric cancer surveillance
Fundic gland polyps (FGPs) are found in up to 90% of patients with FAP.[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2.
https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7
http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
Unlike sporadic FGPs, focal low-grade dysplasia can occur but rarely develops into adenocarcinoma.[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2.
https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7
http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
Gastric adenomas are less common than FGPs in patients with FAP. The risk of gastric adenomas and gastric cancer in patients with FAP appears to be higher in those from geographic areas with high gastric cancer risk.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2.
https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7
http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
Lifetime risk for gastric cancer in patients with FAP or attenuated FAP is reported to be in the range of 0.1% to 7.1%.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
[Figure caption and citation for the preceding image starts]: Fundic gland polypsFrom the personal collection of Lisa A. Boardman, MD; used with permission [Citation ends].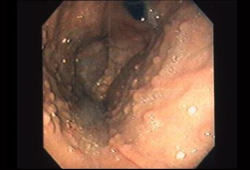
Upper endoscopic surveillance should be carried out at the same time as duodenal/periampullary surveillance (i.e., starting at age 20-30 years), with surveillance intervals determined by the Spigelman stage of duodenal polyposis.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62.
http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com
Gastric cancer risk may be elevated in the setting of certain endoscopic findings, including carpeting of FGPs, polyps >10 mm to 20 mm, mounds of polyps, and proximal gastric white mucosal patches.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2.
https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7
http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
Random sampling of FGPs should be undertaken and surgery reserved for high-grade dysplasia or cancer.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62.
http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com
Presence of FGPs with low-grade dysplasia alone, in the absence of high-risk features, does not require specialized surveillance. High-risk histologic features include tubular adenomas, polyps with high-grade dysplasia, and pyloric gland adenomas.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
Patients with high-risk lesions that cannot be removed endoscopically should be referred to a specialized center for consideration of gastrectomy.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
Small bowel screening[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2.
https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7
http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
[86]Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2015 Apr;47(4):352-76.
https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0034-1391855
http://www.ncbi.nlm.nih.gov/pubmed/25826168?tool=bestpractice.com
The need for small bowel polyp screening beyond the duodenum is controversial in FAP. Small bowel surveillance may be discussed with patients with FAP and may be considered, particularly in the setting of obstructive symptoms or a family history of small intestinal polyposis in other family members.
Options for imaging include small bowel enteroclysis, CT or MR enterography, or video capsule endoscopy.
Thyroid screening
Thyroid cancer occurs in 1% to 2% of the FAP population, compared with 0.2% of the general population. Most cases occur in women.[28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27.
https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
Guidelines differ with regard to recommendations for thyroid screening, although most recommend annual ultrasound of the thyroid gland, with or without physical exam.[28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27.
https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62.
http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com
[40]Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 Oct 1;30(10):1558-71.
https://www.sciencedirect.com/science/article/pii/S0923753419609774
http://www.ncbi.nlm.nih.gov/pubmed/31378807?tool=bestpractice.com
European guidelines recommend starting screening at age 25-30 years, or when colorectal polyposis is diagnosed, whichever comes first.[40]Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 Oct 1;30(10):1558-71.
https://www.sciencedirect.com/science/article/pii/S0923753419609774
http://www.ncbi.nlm.nih.gov/pubmed/31378807?tool=bestpractice.com
The NCCN differs in that it recommends a baseline thyroid ultrasound in late adolescence, with a follow-up every 2-5 years. Shorter follow up intervals may be considered for patients with a family history of thyroid cancer.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
Hepatoblastoma screening
Recommendations for hepatoblastoma screening in patients with FAP vary. Some have suggested annual abdominal ultrasound and serum alpha-fetoprotein (AFP) testing for children with a family history of FAP.[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2.
https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7
http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
[40]Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 Oct 1;30(10):1558-71.
https://www.sciencedirect.com/science/article/pii/S0923753419609774
http://www.ncbi.nlm.nih.gov/pubmed/31378807?tool=bestpractice.com
The NCCN suggests liver palpation, abdominal ultrasound, and measurement of AFP every 3-6 months during the first 5 years of life, but the guidelines note that high-level evidence to support this is lacking.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
The European Society for Paediatric Gastroenterology Hepatology and Nutrition guideline recommends against routine screening for hepatoblastoma.[24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41.
https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx
http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com
Medulloblastoma screening
Patients should be educated regarding signs and symptoms of brain cancer and the importance of prompt reporting of abnormal symptoms to their physicians.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
Dermoid tumor surveillance
Patients should be offered immediate abdominal imaging if they have any abdominal symptoms suggestive of desmoid tumors. Consider annual surveillance with abdominal imaging (computed tomography or magnetic resonance imaging) if patients have a history of symptomatic desmoids.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
