Familial adenomatous polyposis syndromes
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
FAP: without colonic adenomas
surveillance colonoscopy
Patients with a family history of familial adenomatous (FAP) who are adenomatous polyposis coli (APC)-positive should undergo surveillance endoscopy to identify and/or monitor colonic adenomas.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [30]Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar;69(3):411-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034349 http://www.ncbi.nlm.nih.gov/pubmed/31780574?tool=bestpractice.com [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com [38]Issaka RB, Chan AT, Gupta S. AGA clinical practice update on risk stratification for colorectal cancer screening and post-polypectomy surveillance: expert review. Gastroenterology. 2023 Nov;165(5):1280-91. https://www.gastrojournal.org/article/S0016-5085(23)04771-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F http://www.ncbi.nlm.nih.gov/pubmed/37737817?tool=bestpractice.com
The National Comprehensive Cancer Network advises that colonoscopy should be offered starting from aged 10 to 15 years. Other guidance, including from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition, recommends that this surveillance can begin slightly later, from age 12 onwards.[24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [30]Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar;69(3):411-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034349 http://www.ncbi.nlm.nih.gov/pubmed/31780574?tool=bestpractice.com [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com
Frequency of colonic surveillance is individualised depending on colonic phenotype, and is typically undertaken between every 1 and 3 years.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com The presence of alarm symptoms such as anaemia, rectal bleeding, increased bowel movements, and mucous discharge should prompt urgent colonoscopy for any patient, regardless of age or colonic phenotype.[24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com
There is no single consensus for the absolute or relative indications for surgery. Surgery is generally recommended following detection of colonic adenomas, but timing must be individualised and depends on the distribution, size, and histology of colorectal polyps.[24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
The American College of Gastroenterologists advise absolute indications for immediate colorectal surgery include documented or suspected colorectal cancer, or significant symptoms related to colonic neoplasia (e.g., gastrointestinal bleeding).[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com Relative indications for surgery include the presence of multiple adenomas >6 mm; a significant increase in adenoma number; the presence of adenoma with high-grade dysplasia; and the inability to adequately survey the colon due to multiple diminutive polyps.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com
The European Hereditary Tumor Group and European Society of Coloproctology recommend indications for immediate colorectal surgery include certain or suspected cancer; severe symptoms from polyposis; severe disease (>1000 polyps identified at colonoscopy); and unfavourable histological features (e.g., villous adenoma).[37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com Indications for planned surgery include polyp >10 mm in diameter; favourable histology features; substantial increase in polyp number between examinations; and sparse disease (100-1000 polyps).[37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com
FAP: with colonic adenomas
total proctocolectomy with ileal pouch-anal anastomosis (IPAA)
Prophylactic proctocolectomy with IPAA is the main means for preventing colorectal cancer in familial adenomatous polyposis (FAP).
There is no single consensus for the absolute or relative indications for surgery. Surgery is generally recommended following detection of colonic adenomas, but timing must be individualised and depends on the distribution, size, and histology of colorectal polyps.[24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
Absolute indications for immediate colorectal surgery in patients include documented or suspected colorectal cancer; significant symptoms related to colonic neoplasia (e.g., gastrointestinal bleeding).[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com Relative indications for surgery include presence of multiple adenomas >6 mm; significant increase in adenoma number; presence of adenoma with high-grade dysplasia; inability to adequately survey the colon due to multiple diminutive polyps.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com
The European Hereditary Tumor Group and European Society of Coloproctology recommend indications for immediate colorectal surgery include certain or suspected cancer; severe symptoms from polyposis; severe disease (>1000 polyps identified at colonoscopy); and unfavourable histological features (e.g., villous adenoma).[37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com Indications for planned surgery include polyp >10 mm in diameter; favourable histology features; substantial increase in polyp number between examinations; and sparse disease (100-1000 polyps).[37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com
IPAA is offered to patients with classical FAP; patients with attenuated FAP with numerous polyps resulting in carpeting of the rectum; those with curable rectal cancer complicating the polyposis; those who have previously undergone ileorectal anastomosis and now have an unstable rectum in terms of polyp number, size, or histology. The procedure is usually not offered to: patients with incurable cancer; those with an intra-abdominal desmoid that may interfere with the completion of surgery; those with anatomical, physiological, or pathological contraindications to an IPAA; where there is concern regarding the ability of patients to participate in close endoscopic surveillance following surgery.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2
The advantages of total proctocolectomy with IPAA are that the risk of developing rectal cancer is negligible and a permanent stoma is not needed. The disadvantages are that it is a complex operation, a temporary stoma is usually needed, and there is a small risk of bladder or sexual dysfunction, and anal sphincter injury. Bowel function, although usually reasonable, is also somewhat unpredictable. Mucosectomy to maximise removal of rectal mucosa may be considered in IPAA.[39]Chambers WM, McC Mortensen NJ. Should ileal pouch-anal anastomosis include mucosectomy? Colorectal Dis. 2007;9:384-392. http://www.ncbi.nlm.nih.gov/pubmed/17504334?tool=bestpractice.com
Hand-sewn or stapled IPAA techniques may be used. Retrospective data from a large, prospective, institutional genetic registry of patients with FAP showed that although stapled IPAA has a higher incidence of anal transitional zone polyps requiring treatment, compared with hand-sewn IPAA, the incidence of anal transitional zone adenocarcinoma was similar between the two groups.[41]Ozdemir Y, Kalady MF, Aytac E, et al. Anal transitional zone neoplasia in patients with familial adenomatous polyposis after restorative proctocolectomy and IPAA: incidence, management, and oncologic and functional outcomes. Dis Colon Rectum. 2013;56:808-814. http://www.ncbi.nlm.nih.gov/pubmed/23739186?tool=bestpractice.com The stapled anastomosis is associated with better long-term bowel function than a hand-sewn anastomosis. It is therefore the preferred technique for the surgical management of patients with FAP in some centres.[41]Ozdemir Y, Kalady MF, Aytac E, et al. Anal transitional zone neoplasia in patients with familial adenomatous polyposis after restorative proctocolectomy and IPAA: incidence, management, and oncologic and functional outcomes. Dis Colon Rectum. 2013;56:808-814. http://www.ncbi.nlm.nih.gov/pubmed/23739186?tool=bestpractice.com
There is evidence that laparoscopic IPAA is a feasible and safe procedure, although large, good-quality trials examining differences between laparoscopic and open approaches in terms of postoperative complications, cosmesis, quality of life, and costs are needed.[42]Xie YQ, Yuan X. Meta analysis of surgical treatment of 231 cases of familial adenomatous polyposis in China. Chin J Cancer Prev Treat. 2008;15:537-540.[43]Ahmed AU, Keus F, Heikens JT, et al. Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst Rev. 2009;(1):CD006267.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006267.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/19160273?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Brooke ileostomy in familial adenomatous polyposis: adenoma riskFrom the personal collection of Lisa A. Boardman, MD; used with permission [Citation ends].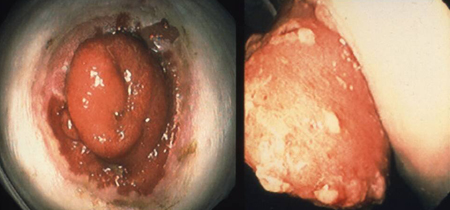
Female patients should be counselled that IPAA is associated with an increase in infertility.[44]Rajaratnam SG, Eglinton TW, Hider P, et al. Impact of ileal pouch-anal anastomosis on female fertility: meta-analysis and systematic review. Int J Colorectal Dis. 2011;26:1365-1374. http://www.ncbi.nlm.nih.gov/pubmed/21766164?tool=bestpractice.com
All patients should be counselled that IPAA requires yearly endoscopic surveillance of the anal transitional zone and pouch to rule out polyp development and to minimise the risk of anal transitional zone cancer.[45]Tudyka VN, Clark SK. Surgical treatment in familial adenomatous polyposis. Ann Gastroenterol. 2012;25(3):201-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959379 http://www.ncbi.nlm.nih.gov/pubmed/24714154?tool=bestpractice.com
chemoprevention
Additional treatment recommended for SOME patients in selected patient group
Chemoprevention may be considered as an adjunct in the management of retained rectum or ileal pouch in select patients following prophylactic surgery.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
Available data suggest that sulindac, a cyclo-oxygenase (COX)-1 and COX-2 inhibitor, is the most potent drug for polyp regression. However, it is not yet known whether decreases in polyp burden reduces colorectal cancer risk.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [60]Cruz-Correa M, Hylind LM, Romans KE, et al. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002 Mar;122(3):641-5. https://www.doi.org/10.1053/gast.2002.31890 http://www.ncbi.nlm.nih.gov/pubmed/11874996?tool=bestpractice.com
Patients should be referred to expert centres for consideration of enrolment in a clinical trial if they are interested in chemoprevention. There are currently no approved drugs for this indication.
total proctocolectomy with ileorectal anastomosis (IRA) or other alternative surgery
Ileal pouch-anal anastomosis (IPAA) may not be possible in patients who have had previous pelvic surgery or who have shortening of the mesentery. Women of childbearing age may also choose IRA over IPAA until after childbearing (as IPAA is associated with an increase in fertility), when they can convert to IPAA.[45]Tudyka VN, Clark SK. Surgical treatment in familial adenomatous polyposis. Ann Gastroenterol. 2012;25(3):201-6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959379
http://www.ncbi.nlm.nih.gov/pubmed/24714154?tool=bestpractice.com
If IPAA is not possible or is to be delayed, then IRA, continent ileostomy, or Brooke ileostomy can be performed.[Figure caption and citation for the preceding image starts]: Brooke ileostomy in familial adenomatous polyposis: adenoma riskFrom the personal collection of Lisa A. Boardman, MD; used with permission [Citation ends].
IRA is an option for patients with familial adenomatous polyposis (FAP) with few or no rectal adenomas.[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2. https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7 http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com Patients who have IRA have better continence and bowel function following surgery, compared with patients having IPAA.[49]Günther K, Braunrieder G, Bittorf BR, et al. Patients with familial adenomatous polyposis experience better bowel function and quality of life after ileorectal anastomosis than after ileoanal pouch. Colorectal Dis. 2003 Jan;5(1):38-44. http://www.ncbi.nlm.nih.gov/pubmed/12780925?tool=bestpractice.com
However, the retained rectal mucosa remains at risk from polyp formation, and patients must undergo close endoscopic observation of the remaining rectum. In one study, 13% of patients with FAP who underwent IRA developed rectal cancer a median of 10 years after surgery.[46]Jenner DC, Levitt S. Rectal cancer following colectomy and ileorectal anastomosis for familial adenomatous polyposis. Aust N Z J Surg. 1998;68:136-138. http://www.ncbi.nlm.nih.gov/pubmed/9494007?tool=bestpractice.com The risk of cancer development in the rectal stump rises considerably after age 50 years. If the rectum becomes unstable, a proctectomy with either an IPAA or end ileostomy is recommended.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2
chemoprevention
Additional treatment recommended for SOME patients in selected patient group
Chemoprevention may be considered as an adjunct in the management of retained rectum or ileal pouch in select patients following prophylactic surgery.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
Available data suggest that sulindac, a cyclo-oxygenase (COX)-1 and COX-2 inhibitor, is the most potent drug for polyp regression. However, it is not yet known whether decreases in polyp burden reduces colorectal cancer risk.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [60]Cruz-Correa M, Hylind LM, Romans KE, et al. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002 Mar;122(3):641-5. https://www.doi.org/10.1053/gast.2002.31890 http://www.ncbi.nlm.nih.gov/pubmed/11874996?tool=bestpractice.com
Patients should be referred to expert centres for consideration of enrolment in a clinical trial if they are interested in chemoprevention. There are currently no approved drugs for this indication.
pharmacotherapy, radiotherapy, or surgery
Treatment recommended for ALL patients in selected patient group
Patients with familial adenomatous polyposis (FAP) and desmoid tumours should be referred to centres specialising in management of desmoid tumours.
Pharmacological therapy is usually the preferred initial approach.[28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com Surgery can stimulate intra-abdominal desmoid tumour growth in patients with FAP and is only typically offered to symptomatic patients who do not respond to initial medical therapy.[28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
Available treatments include cyclo-oxygenase (COX)-2 inhibitors (e.g., celecoxib) or combined COX-1 and COX-2 inhibitors (e.g., sulindac), antiestrogens (e.g., tamoxifen), chemotherapy, radiotherapy, and surgery. Imatinib has been associated with both partial response and growth stabilisation, even in desmoids that do not have apparent mutations in the KIT tyrosine kinase oncogene.[53]Duffaud F, Le Cesne A. Imatinib in the treatment of solid tumors. Targ Oncol. 2009;4:45-56. http://www.ncbi.nlm.nih.gov/pubmed/19343301?tool=bestpractice.com However, the efficacy of these various treatment modalities is unclear given there are no controlled trials comparing them. Treatment decisions should be made in consultation with an oncologist.
Complicating our understanding of the impact of these treatments is the phenomenon of spontaneous regression of desmoids, which may occur in up to 10% of cases.[54]Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg. 1996;83:1494-1504. http://www.ncbi.nlm.nih.gov/pubmed/9014661?tool=bestpractice.com [55]Knudsen AL, Bulow S. Desmoid tumour in familial adenomatous polyposis: a review of literature. Fam Cancer. 2001;1:111-119. http://www.ncbi.nlm.nih.gov/pubmed/14574007?tool=bestpractice.com
Choice of treatment for desmoid tumours must be guided by whether the patient is receiving chemoprevention in the management of FAP, and the drug(s) already being used.
surveillance endoscopy with treatment depending on polyp burden
Treatment recommended for ALL patients in selected patient group
At-risk patients found to have colorectal adenomas, as well as known adenomatous polyposis coli gene carriers, should have forward- and side-viewing oesophagogastroduodenoscopies at least every 5 years.
The American Society of Colon and Rectal Surgeons and the National Comprehensive Cancer Network recommend that screening for duodenal adenomas begin at aged 20-25 years, although this may be offered to younger patients if there is a family history of duodenal cancer or significant duodenal polyp burden.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com [30]Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar;69(3):411-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034349 http://www.ncbi.nlm.nih.gov/pubmed/31780574?tool=bestpractice.com The American College of Gastroenterologists and the European Society of Medical Oncology advise a later starting age of 25-30 years.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com [30]Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar;69(3):411-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034349 http://www.ncbi.nlm.nih.gov/pubmed/31780574?tool=bestpractice.com [56]Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 Oct 1;30(10):1558-71. https://www.sciencedirect.com/science/article/pii/S0923753419609774 http://www.ncbi.nlm.nih.gov/pubmed/31378807?tool=bestpractice.com Endoscopy should be repeated every 4-5 years until polyps are found.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com [56]Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 Oct 1;30(10):1558-71. https://www.sciencedirect.com/science/article/pii/S0923753419609774 http://www.ncbi.nlm.nih.gov/pubmed/31378807?tool=bestpractice.com
Once polyps develop, the Spigelman criteria can be used to determine the appropriate surveillance intervals:[36]Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989 Sep 30;2(8666):783-5. http://www.ncbi.nlm.nih.gov/pubmed/2571019?tool=bestpractice.com
Stage 0: 3-5 years
Stage 1: 2-3 years
Stage 2: 1-2 years
Stage 3: 6-12 months
Stage 4: surveillance should occur every 3-6 months along with surgical consultation for consideration of duodenectomy.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2
Patients with advanced polyposis should be referred to expert centres to be managed by endoscopists with expertise in familial adenomatous polyposis.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 Endoscopic treatment is used for downstaging, with the aim of delaying the development of stage 4 disease. Endoscopic therapies for duodenal adenomas include polypectomy, endoscopic mucosal resection, and ablation.[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2. https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7 http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
There is insufficient evidence for the benefit of chemoprevention in the management of duodenal polyps. Patients should be referred to expert centres for consideration of enrolment in a clinical trial if they are interested in chemoprevention. There are currently no approved drugs for the prevention or regression of duodenal adenomas.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2
attenuated FAP: without colonic adenomas
surveillance colonoscopy
Patients with a family history of attenuated familial adenomatous polyposis who are adenomatous polyposis coli (APC)-positive should undergo surveillance endoscopy to identify colonic adenomas.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [30]Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar;69(3):411-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034349 http://www.ncbi.nlm.nih.gov/pubmed/31780574?tool=bestpractice.com [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com
Surveillance should begin in the late teens, and generally no later than 20 years age.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com Frequency of colonic surveillance is individualised depending on colonic phenotype, and is typically undertaken between every 1 and 3 years.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com
The presence of alarm symptoms such as anaemia, rectal bleeding, increased bowel movements, and mucous discharge should prompt urgent colonoscopy for any patient, regardless of age or colonic phenotype.[24]Hyer W, Cohen S, Attard T, et al. Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019 Mar;68(3):428-41. https://journals.lww.com/jpgn/Fulltext/2019/03000/Management_of_Familial_Adenomatous_Polyposis_in.30.aspx http://www.ncbi.nlm.nih.gov/pubmed/30585891?tool=bestpractice.com [37]Zaffaroni G, Mannucci A, Koskenvuo L, et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: a joint EHTG-ESCP revision. Br J Surg. 2024 May 3;111(5):znae070. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11081080 http://www.ncbi.nlm.nih.gov/pubmed/38722804?tool=bestpractice.com
attenuated FAP: with colonic adenomas
surveillance colonoscopy with polyp clearance
Frequent colonoscopic adenoma clearance may be suitable for patients with a low polyp burden.[40]Bjork JA, Akerbrant HI, Iselius LE, et al. Risk factors for rectal cancer morbidity and mortality in patients with familial adenomatous polyposis after colectomy and ileorectal anastomosis. Dis Colon Rectum. 2000;43:1719-1725. http://www.ncbi.nlm.nih.gov/pubmed/11156457?tool=bestpractice.com [48]van Leerdam ME, Roos VH, van Hooft JE, et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019 Sep;51(9):877-895. https://www.doi.org/10.1055/a-0965-0605 http://www.ncbi.nlm.nih.gov/pubmed/31342472?tool=bestpractice.com [57]Nugent KP, Phillips RK. Rectal cancer risk in older patients with familial adenomatous polyposis and an ileorectal anastomosis: a cause for concern. Br J Surg. 1992;79:1204-1206. http://www.ncbi.nlm.nih.gov/pubmed/1334761?tool=bestpractice.com [58]Parc YR, Olschwang S, Desaint B, et al. Familial adenomatous polyposis: prevalence of adenomas in the ileal pouch after restorative proctocolectomy. Ann Surg. 2001 Mar;233(3):360-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1421251 http://www.ncbi.nlm.nih.gov/pubmed/11224623?tool=bestpractice.com [59]van Duijvendijk P, Vasen HF, Bertario L, et al. Cumulative risk of developing polyps or malignancy at the ileal pouch-anal anastomosis in patients with familial adenomatous polyposis. J Gastrointest Surg. 1999;3:325-330. http://www.ncbi.nlm.nih.gov/pubmed/10481126?tool=bestpractice.com This is generally defined as having <20 adenomas (all <1 cm in diameter) with no advanced histology.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2
ileal pouch-anal anastomosis (IPAA) or ileorectal anastomosis (IRA)
Surgery should be offered where adenoma burden cannot be managed endoscopically. This is typically when polyp burden is >20 (at any individual examination), polyps have been previously ablated, some polyps are >1 cm in size, or advanced histology is noted in any polyp.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2
Choice of surgery in attenuated familial adenomatous polyposis is individualised based on the age of the patients, their previous surgical history, location of polyps, and patient preferences.[48]van Leerdam ME, Roos VH, van Hooft JE, et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019 Sep;51(9):877-895. https://www.doi.org/10.1055/a-0965-0605 http://www.ncbi.nlm.nih.gov/pubmed/31342472?tool=bestpractice.com In most cases, rectal adenomas are few, therefore, colectomy with an IRA and continuous, lifelong surveillance of the rectum is the management of choice.
Female patients should be counselled that IPAA is associated with an increase in infertility.[44]Rajaratnam SG, Eglinton TW, Hider P, et al. Impact of ileal pouch-anal anastomosis on female fertility: meta-analysis and systematic review. Int J Colorectal Dis. 2011;26:1365-1374. http://www.ncbi.nlm.nih.gov/pubmed/21766164?tool=bestpractice.com
Rectoscopy or pouchoscopy should be performed at least annually.
chemoprevention
Additional treatment recommended for SOME patients in selected patient group
Chemoprevention may be considered as an adjunct in the management of retained rectum or ileal pouch in select patients following prophylactic surgery.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
Available data suggest that sulindac, a cyclo-oxygenase (COX)-1 and COX-2 inhibitor, is the most potent drug for polyp regression. However, it is not yet known whether decreases in polyp burden reduces colorectal cancer risk.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [60]Cruz-Correa M, Hylind LM, Romans KE, et al. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002 Mar;122(3):641-5. https://www.doi.org/10.1053/gast.2002.31890 http://www.ncbi.nlm.nih.gov/pubmed/11874996?tool=bestpractice.com
Patients should be referred to expert centres for consideration of enrolment in a clinical trial if they are interested in chemoprevention. There are currently no approved drugs for this indication.
pharmacotherapy, radiotherapy, or surgery
Treatment recommended for ALL patients in selected patient group
Patients with attenuated familial adenomatous polyposis (FAP) and desmoid tumours should be referred to centres specialising in management of desmoid tumours.
Pharmacological therapy is usually the preferred initial approach.[28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com Surgery can stimulate intra-abdominal desmoid tumour growth in patients with FAP and is only typically offered to symptomatic patients who do not respond to initial medical therapy.[28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com
Available treatments include cyclo-oxygenase (COX)-2 inhibitors (e.g., celecoxib) or combined COX-1 and COX-2 inhibitors (e.g., sulindac), anti-oestrogens (e.g., tamoxifen), chemotherapy and radiotherapy, and surgery. Imatinib has been associated with both partial response and growth stabilisation, even in desmoids that do not have apparent mutations in the KIT tyrosine kinase oncogene.[53]Duffaud F, Le Cesne A. Imatinib in the treatment of solid tumors. Targ Oncol. 2009;4:45-56. http://www.ncbi.nlm.nih.gov/pubmed/19343301?tool=bestpractice.com However, the efficacy of these various treatment modalities is unclear given there are no controlled trials comparing them. Treatment decisions should be made in consultation with an oncologist.
Complicating our understanding of the impact of these treatments is the phenomenon of spontaneous regression of desmoids, which may occur in up to 10% of cases.[54]Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg. 1996;83:1494-1504. http://www.ncbi.nlm.nih.gov/pubmed/9014661?tool=bestpractice.com [55]Knudsen AL, Bulow S. Desmoid tumour in familial adenomatous polyposis: a review of literature. Fam Cancer. 2001;1:111-119. http://www.ncbi.nlm.nih.gov/pubmed/14574007?tool=bestpractice.com
Choice of treatment for desmoid tumours must be guided by whether the patient is receiving chemoprevention in the management of FAP, and the drug(s) already being used.
surveillance endoscopy with treatment depending on polyp burden
Treatment recommended for ALL patients in selected patient group
At-risk patients found to have colorectal adenomas, as well as known adenomatous polyposis coli gene carriers, should have forward- and side-viewing oesophagogastroduodenoscopies at least every 5 years.
The American Society of Colon and Rectal Surgeons and the National Comprehensive Cancer Network recommend that screening for duodenal adenomas begin at aged 20-25 years, although this may be offered to younger patients if there is a family history of duodenal cancer or significant duodenal polyp burden.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 [28]Poylin VY, Shaffer VO, Felder SI, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited adenomatous polyposis syndromes. Dis Colon Rectum. 2024 Feb 1;67(2):213-27. https://journals.lww.com/dcrjournal/fulltext/2024/02000/the_american_society_of_colon_and_rectal_surgeons.6.aspx http://www.ncbi.nlm.nih.gov/pubmed/37682806?tool=bestpractice.com [30]Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar;69(3):411-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034349 http://www.ncbi.nlm.nih.gov/pubmed/31780574?tool=bestpractice.com The American College of Gastroenterologists and the European Society of Medical Oncology advise a later starting age of 25-30 years.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com [30]Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar;69(3):411-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034349 http://www.ncbi.nlm.nih.gov/pubmed/31780574?tool=bestpractice.com [56]Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 Oct 1;30(10):1558-71. https://www.sciencedirect.com/science/article/pii/S0923753419609774 http://www.ncbi.nlm.nih.gov/pubmed/31378807?tool=bestpractice.com Endoscopy should be repeated every 4-5 years until polyps are found.[29]Syngal S, Brand RE, Church JM, et al.; American College of Gastroenterology. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62. http://www.ncbi.nlm.nih.gov/pubmed/25645574?tool=bestpractice.com [56]Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 Oct 1;30(10):1558-71. https://www.sciencedirect.com/science/article/pii/S0923753419609774 http://www.ncbi.nlm.nih.gov/pubmed/31378807?tool=bestpractice.com
Once polyps develop, the Spigelman criteria can be used to determine the appropriate surveillance intervals:[36]Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989 Sep 30;2(8666):783-5. http://www.ncbi.nlm.nih.gov/pubmed/2571019?tool=bestpractice.com
Stage 0: 3-5 years
Stage 1: 2-3 years
Stage 2: 1-2 years
Stage 3: 6-12 months
Stage 4: surveillance should occur every 3-6 months along with surgical consultation for consideration of duodenectomy.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2
Patients with advanced polyposis should be referred to expert centers to be managed by endoscopists with expertise in familial adenomatous polyposis.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2 Endoscopic treatment is used for downstaging, with the aim of delaying the development of stage 4 disease. Endoscopic therapies for duodenal adenomas include polypectomy, endoscopic mucosal resection, and ablation.[12]Yang J, Gurudu SR, Koptiuch C, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May;91(5):963-82.e2. https://linkinghub.elsevier.com/retrieve/pii/S0016-5107(20)30054-7 http://www.ncbi.nlm.nih.gov/pubmed/32169282?tool=bestpractice.com
There is insufficient evidence for the benefit of chemoprevention in the management of duodenal polyps. Patients should be referred to expert centres for consideration of enrolment in a clinical trial if they are interested in chemoprevention. There are currently no approved drugs for the prevention or regression of duodenal adenomas.[9]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication]. https://www.nccn.org/guidelines/category_2

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer