Etiology
Ventricular tachycardia (VT) is usually observed in the setting of ischemic heart disease or underlying nonischemic cardiomyopathy, although it may also be observed in patients without structural heart disease (idiopathic VT). Ischemia and coronary artery disease (CAD) are the most common etiologies of VT around the world, especially among North Americans and Europeans. Across the developing world, infectious and other forms of nonischemic cardiomyopathy may also play a significant role in the etiology of ventricular arrhythmias (e.g., Chagas disease in Central America).[3]
Other forms of structural heart disease, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and anomalous coronary arteries are also associated with ventricular arrhythmias. At the cellular level, congenital or acquired abnormalities in cardiac sodium channels (congenital long QT syndrome, Brugada syndrome), potassium channels (long QT syndrome, short QT syndrome), and calcium channels (catecholaminergic polymorphic VT) have been implicated as causes of VT and sudden death. Mutations in cardiac anchoring proteins may also lead to the long QT syndrome.[4]
See Hypertrophic cardiomyopathy and Long QT syndrome for more information.
Environmental and/or physiologic stressors can unmask subclinical abnormalities that predispose to sudden cardiac death. Pharmacogenetic variants exist that result in prolongation of the QT interval with administration of certain drugs (an up-to-date list of drugs is available through research centers CredibleMeds: Arizona Center for Education and Research on Therapeutics Opens in new window); catecholamine-sensitive VT may only arise during periods of heightened mental or physical stress. It is estimated that 5% to 10% of sudden cardiac deaths occur in the absence of cardiomyopathy or CAD.[5]
Pathophysiology
Among patients with prior myocardial infarction or nonischemic cardiomyopathy, regions of slowed conduction (usually adjacent to damaged myocardium or scar) are the substrate for reentrant arrhythmias. Ventricular tachycardia (VT) may also arise from triggered activity due to early after-depolarizations (EADs) leading to torsades de pointes, a polymorphic VT seen in the setting of a prolonged QT interval, or delayed after-depolarizations (DADs), which are seen in idiopathic right ventricular outflow tract VT (or its variants, from other locations) or catecholaminergic polymorphic VT.[6] EADs occur during phase 2 or 3 of the action potential, whereas DADs occur during phase 4. When an EAD or DAD reaches a "threshold" potential, it can result in triggering of another action potential. Enhanced automaticity of the ventricular tissue may also produce a relatively slow VT called accelerated idioventricular rhythm, which generally follows a benign course. This arrhythmia is frequently observed in the setting of acute ischemia or early after reperfusion.[7]
Classification
Ventricular tachycardia (VT)
Presence of a wide complex tachycardia (QRS 120 milliseconds or greater) at a rate of 100 bpm or greater.
Sustained VT
A ventricular rhythm faster than 100 bpm typically lasting at least 30 seconds or requiring termination due to hemodynamic instability. [Figure caption and citation for the preceding image starts]: Sustained (monomorphic) ventricular tachycardiaFrom the collection of Prof Sei Iwai; used with permission [Citation ends].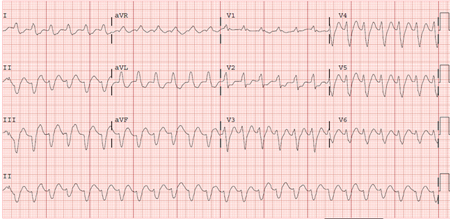
Nonsustained VT
A ventricular rhythm faster than 100 bpm lasting for at least 3 consecutive beats but terminating spontaneously in less than 30 seconds, and not resulting in significant hemodynamic instability.
Polymorphic VT
Tachyarrhythmia with multiple different wide QRS complex (>120 milliseconds) morphologies arising from the ventricles.
Monomorphic VT
Tachyarrhythmia with an organized, single-morphology QRS complex arising from one of the ventricles.
Bidirectional VT
VT with a beat-to-beat alternation in QRS axis, classically seen in digitalis toxicity or catecholaminergic polymorphic VT.
Hemodynamically stable VT
VT associated with a normal blood pressure and no symptoms due to hemodynamic compromise.
Hemodynamically unstable VT
VT associated with hypotension, signs of diminished cerebral perfusion (e.g., confusion, dizziness, syncope), or signs of diminished coronary perfusion (e.g., angina, dyspnea).
Idiopathic VT
VT occurring in the absence of apparent structural heart disease (e.g., ischemia, prior infarction, cardiomyopathy, valvular disease, arrhythmogenic right ventricular cardiomyopathy, sarcoid, left ventricular noncompaction, or other disorders of the myocardium), known channelopathy (e.g., long QT syndrome, Brugada syndrome, catecholaminergic polymorphic VT, short QT syndrome), drug toxicity, or electrolyte imbalance. [Figure caption and citation for the preceding image starts]: Right ventricular outflow tract tachycardiaFrom the collection of Prof Sei Iwai; used with permission [Citation ends].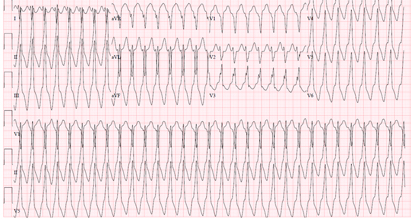
Torsades de pointes (TdP)
A form of polymorphic VT with a characteristic twisting morphology occurring in the setting of QT interval prolongation. TdP is initiated by an early after-depolarization and perpetuated by reentry. [Figure caption and citation for the preceding image starts]: Torsades de pointesFrom the collections of Dr Kenneth Stein and Dr Richard Keating; used with permission [Citation ends].
Catecholaminergic polymorphic VT
Patients with this condition demonstrate cellular abnormalities of calcium handling, especially during periods of sympathetic stimulation. Increased intracellular calcium predisposes the patients to develop VT.
Outflow tract VT
This form of "idiopathic" VT was originally described as arising from the right ventricular outflow tract, but may also originate from other areas including the left ventricular outflow tract region (including the sinuses of Valsalva, left fibrous trigone, left ventricular summit, and tricuspid and mitral annuli), and likely results from cyclic adenosine monophosphate-mediated triggered activity; therefore, this form of VT is uniquely sensitive to adenosine.
Fascicular VT
A common form of idiopathic VT arising from the left ventricle, with reentrant circuit partially involving the Purkinje fibers, that is characteristically sensitive to verapamil.
Use of this content is subject to our disclaimer