Approach
Diagnostic testing for gonorrhea should be considered with any genitourinary symptom in sexually active people: in particular, men who have dysuria, urethral irritation, or urethral discharge, and women with vaginal discharge, pelvic pain or suspected pelvic inflammatory disease (PID), and tubo-ovarian abscess. Mothers of newborns with ophthalmia neonatorum, scalp abscesses, or disseminated gonococcal infections, and their sex partners, should be evaluated and tested.[23] Neonates who are born to women with untreated gonococcal infections are at high risk of infections and should be tested at exposed sites (e.g., conjunctiva, vagina, rectum, and oropharynx).[23]
History
Eliciting a history of sexual activity and risk factors is important when considering the diagnosis of gonorrhea. A sexual history must be included in any assessment of genitourinary symptoms or for patients with systemic symptoms that may be uncommon presentations of gonorrhea, such as arthritis, meningitis, or endocarditis. At-risk populations (e.g., women ages 15-24 years, and men ages 20-29 years) can be prompted about sexual history as an important prevention measure for their age group.
Important elements of the sexual history include the 5 Ps:
Partners (sex of partners, number of partners in prior 2 months/1 year)
Prevention of pregnancy - trying to conceive or contraceptive use
Protection from STIs/HIV - what does the patient do to protect him/herself from STIs and HIV?
Practices or types of sexual activities (oral/vaginal/anal and insertive/receptive), condom use
Past STIs/HIV - any prior diagnoses of STIs or HIV or viral hepatitis.
In men, the presenting complaint may be a mucopurulent or purulent urethral discharge. However, if this is not present, other symptoms that suggest urethritis are dysuria and urethral pruritus. Urethritis can be asymptomatic. Frequency and urgency are typically absent in urethritis. Symptoms of prostatitis include pain in the lower back and genital area, urinary frequency and urgency (often at night), and burning or painful urination.
In women, signs and symptoms to enquire about are vaginal discharge, pelvic pain, or fever. Women with gonorrhea may have some vaginal discharge, but lack of a discharge does not exclude infection.
Gonorrhea infections in the rectal area are common in men who have sex with men (MSM), but perianal contamination from a cervical infection or a direct infection from anal intercourse can cause anorectal infections in women. Symptoms include anal pruritus and mucopurulent discharge, usually with a bowel movement. Rectal pain, tenesmus, and bleeding are more common in MSM.
To help with treatment planning, patients should be asked about any known allergies to antibiotics, particularly penicillin (immunoglobulin E-mediated), cephalosporins, and azithromycin. An infectious disease specialist should be consulted if there is known antibiotic allergy.
Physical exam
Male genital tract
Inspection and palpation of the testis, epididymis, and spermatic cord should be performed. The penis shaft, glans, and meatus should also be examined and the presence of discharge assessed. A prostate exam is required if symptoms of prostatitis are present.
A swollen and/or tender testicle (usually one-sided) on palpation may indicate orchitis.
A swollen and/or tender epididymis on palpation may indicate epididymitis, which occurs in <5% of men with gonorrhea.[40] Unilateral testicular pain (without discharge or dysuria) and fever are also symptoms of epididymitis.[2]
Female genital tract
The external genitalia (labia and clitoris) should be inspected before speculum exam of the cervix and vagina. It is recommended that lubricant jelly not be used as it can destroy Neisseria gonorrhoeae. Presence of mucopurulent or purulent exudate at the endocervix is looked for.
PID is the most important complication of gonorrhea in women. It may develop in up to one third of women with gonorrhea, and can lead to long-term sequelae even after resolution of infection.[41][42] The most common sequelae of PID are chronic pelvic pain (40%), tubal infertility (10.8%), and ectopic pregnancy (9.1%).[43][44] A bimanual exam for cervical motion tenderness, uterine tenderness, and adnexal tenderness is particularly important to assess possible ascending infection resulting in PID. Cervical motion tenderness is assessed using 1 or 2 fingertips to move the cervix and asking the patient if any pain results. Presence of a cervical mass suggests PID.
Bleeding that occurs with gentle passage of a cotton swab through the cervical os suggests cervical friability and cervicitis.[23]
Extragenital infection
Rectal gonorrhea may cause mucopurulent discharge from the anus.
The pharynx is examined for erythema and exudate.[Figure caption and citation for the preceding image starts]: This patient presented with symptoms later diagnosed as due to gonococcal pharyngitisCDC/ Dr N. J. Flumara, Dr Gavin Hart [Citation ends].
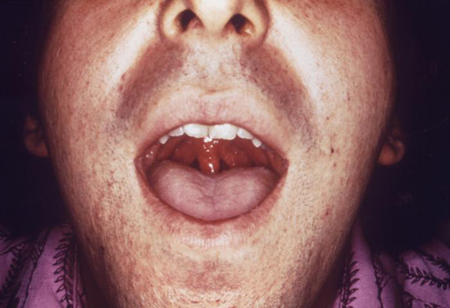 Anterior cervical lymphadenopathy may also be present.[2]
Anterior cervical lymphadenopathy may also be present.[2]Gonococcal conjunctivitis can present with a thick white/yellow discharge. Examination of the eyes with a slit lamp is recommended so that infection of the cornea can be excluded.
Suspected disseminated gonococcal infection (DGI)
DGI occurs when infection with N gonorrhoeae is left untreated. It occurs in <3% of gonorrhea infections.[45] Women are thought to be more likely to develop DGI than men, possibly related to menses. It commonly causes skin (75%) and synovium (68%) infections. Fever occurs in 60% of patients with DGI. Rare complications include endocarditis, meningitis, myocarditis, and perihepatic abscesses. Patients with DGI often do not present with urogenital symptoms.
Clinical findings include papules that progress into hemorrhagic pustules, bullae, petechiae, or necrotic lesions on the extremities; slight joint pain; or severe polyarthritis, which if left untreated will develop into septic arthritis.[Figure caption and citation for the preceding image starts]: Gonococcal arthritic patient with inflammation of the skin of her right arm due to a disseminated Neisseria gonorrhoeae bacterial infectionCDC/ Emory [Citation ends]. [Figure caption and citation for the preceding image starts]: Gonococcal arthritis of the hand, which caused the hand and wrist to swellCDC/ Susan Lindsley, VD [Citation ends].
[Figure caption and citation for the preceding image starts]: Gonococcal arthritis of the hand, which caused the hand and wrist to swellCDC/ Susan Lindsley, VD [Citation ends].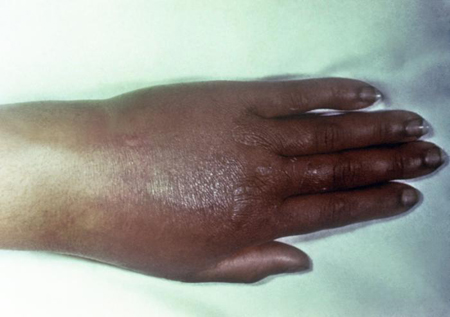 [Figure caption and citation for the preceding image starts]: Cutaneous lesions on the left ankle and calf due to a disseminated Neisseria gonorrhoeae infectionCDC/ Dr S. E. Thompson, VDCD/ J. Pledger [Citation ends].
[Figure caption and citation for the preceding image starts]: Cutaneous lesions on the left ankle and calf due to a disseminated Neisseria gonorrhoeae infectionCDC/ Dr S. E. Thompson, VDCD/ J. Pledger [Citation ends].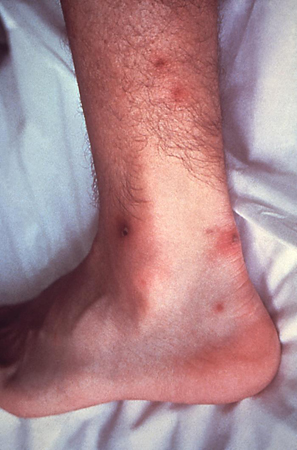 Commonly affected joints are the wrists, ankles, hands, and feet, and initial joint aspiration may be negative for infection. If septic arthritis develops, the commonly involved joints are elbows, wrists, knees, or ankles. Joint aspiration in this case will detect >40,000 leukocytes per mm³ and contain gram-negative intracellular diplococci. Synovial fluid sent for culture usually comes back negative.[2]
Commonly affected joints are the wrists, ankles, hands, and feet, and initial joint aspiration may be negative for infection. If septic arthritis develops, the commonly involved joints are elbows, wrists, knees, or ankles. Joint aspiration in this case will detect >40,000 leukocytes per mm³ and contain gram-negative intracellular diplococci. Synovial fluid sent for culture usually comes back negative.[2]
The patient may also present with sepsis. Gonococcal meningitis will present like other bacterial meninges infections with positive Brudzinski and Kernig signs, purpuric rash, seizures, signs of increased cerebrospinal fluid (CSF) pressure (hypertension with bradycardia), and focal cerebral signs. Gonococcal endocarditis can present with systemic manifestations such as fever, cardiac manifestations such as murmurs, and extracardiac manifestations such as embolic events.
Pediatric gonococcal infection
Infants
The most severe manifestations of pediatric gonococcal infection are ophthalmia neonatorum and sepsis, which can include arthritis and meningitis. Less severe manifestations include rhinitis, vaginitis, urethritis, and reinfection at site of fetal monitoring: for example, through scalp electrodes.[Figure caption and citation for the preceding image starts]: A newborn with gonococcal ophthalmia neonatorum caused by a maternally transmitted gonococcal infectionCDC/ J. Pledger [Citation ends].
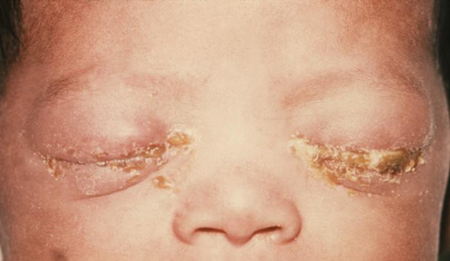
Infants at increased risk for gonococcal ophthalmia neonatorum are those who do not receive ophthalmia prophylaxis and those whose mothers have had no prenatal care, or have a history of STIs or substance misuse.[23] In the US, routine prophylactic eye drops are recommended for all newborns.[38][46]
Children
Sexual abuse is the most frequent cause of STIs (including gonococcal infection) in preadolescent children.[14] Anorectal and pharyngeal infection are common and frequently asymptomatic in sexually abused children. Vaginitis is the most common manifestation in preadolescent girls.
Laboratory evaluation: overview
Nucleic acid amplification tests (NAATs), culture, Gram stain, and urinalysis form the mainstay of diagnostic laboratory tests for N gonorrhoeae. There is no clinically available serologic test for N gonorrhoeae.[47] The order of tests initially depends on patient's sex, whether the patient is symptomatic or asymptomatic, and which site(s) is involved (e.g., genitalia, pharynx, rectum, or conjunctivae).
NAATs are generally available and approved for most anatomic sites; the use of self-collected specimens is also very useful if patients are given proper instructions.[23][48][49] The Association of Public Health Laboratories and the Centers for Disease Control and Prevention recommend NAATs for the detection of genital tract gonorrhea infections without routine repeat testing for positive results.[47][50] An NAAT of urine or genital specimens is preferable to culture in most settings due to better sensitivity.[50] It is also more convenient as samples can be collected by the clinician/healthcare provider or the patient (self-collected).[23] Self-collected specimens sent for NAATs have been found to be noninferior to clinician-collected specimens, although local laboratory validation of this collection method should be conducted.[49][51][52] If available through a local laboratory, NAATs can be used for pharyngeal and rectal specimens, but otherwise culture is the only Food and Drug Administration (FDA)-approved method for detection at nongenital sites. Individual laboratories must pursue verification studies and satisfy regulations for Clinical Laboratory Improvement Amendments (CLIA) compliance in order to perform NAATs for nongenital sites; therefore, this option may not be widely available. When considering NAAT screening, it is important to adhere to guidelines on risk groups because imperfect specificity allows the possibility of false-positive results. This can result in lower positive predictive values in very low prevalence populations. If there is a positive result in someone with very low suspicion from clinical history, repeat testing with an alternative NAAT or culture would be ideal but should not prevent treatment. This is particularly an issue with nongenital sites because mixed flora and inhibitors that can accompany specimens can further impair the specificity of NAATs for gonorrhea.[47]
Culture has been the definitive diagnostic test and is the only way to assess for antimicrobial sensitivity. Culture is reasonably sensitive and highly specific. It is cheap and suitable for use with different types of specimens, and allows retention of the isolate for additional testing such as for antimicrobial sensitivities. Disadvantages are that the specimen must be transported under conditions that are adequate to maintain the viability of the organisms.[47] Culture also has deficiencies in sensitivity with pharyngeal and rectal anal sites (sensitivity of pharynx culture is around 50%).
Gram stain is used for male urethral discharge only as Gram stain of endocervical, pharyngeal, or rectal specimens is not considered sufficient to detect infection; however, it is insufficient for ruling out infection in asymptomatic men.[23][Figure caption and citation for the preceding image starts]: Photomicrograph revealing the histopathology in an acute case of gonococcal urethritis using gram-stain techniqueCDC/ Joe Millar [Citation ends].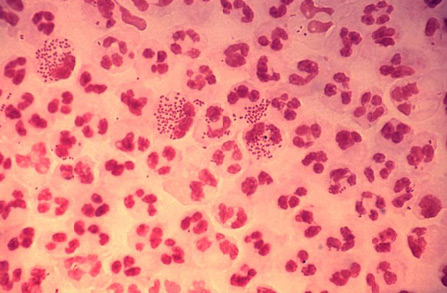
Non-polymerase chain reaction (PCR)-based point-of-care tests for gonorrhea have limited sensitivity and are not recommended for routine use.[53][54][55] Newer near point-of-care or point-of-care PCR assays where results are available within one hour can provide faster turnaround and avoid unnecessary treatment.
It is recommended that all patients who are being tested for gonorrhea also be considered for other STI testing including chlamydia, syphilis, and HIV.[23]
Laboratory evaluation: men with urethral discharge
Gram stain of the discharge is recommended as the initial test because it has high specificity (>99%) and sensitivity (>95%).[23] The test is considered highly suggestive for infection with N gonorrhoeae in symptomatic men if the smear contains typical gram-negative diplococci within polymorphonuclear leukocytes. It is suggested that if possible all men have a confirmatory laboratory test (i.e., culture of urethral discharge, NAAT of urethral swab or urine) following this procedure.
Laboratory evaluation: men without urethral discharge
Urinalysis of a first-void urine specimen can be performed. A presumptive diagnosis of urethritis can be made if the urinalysis strip is positive for leukocyte esterase.
Urine can also be sent for Gram stain; the presence of leukocytes with ≥10 white blood cell (WBC) count per high-power field or ≥2 WBC count per oil immersion field suggests urethritis. The presence of intracellular gram-negative diplococci has a high sensitivity for gonorrhea, although the lack of diplococci should not stop further testing for gonorrhea if the patient has signs of urethritis.[23] A definitive diagnosis can be obtained by NAAT of the urine or urethral swab.[47] Gonorrhea screening for asymptomatic men may also be done by a urine NAAT alone with no urinalysis.
For men who are asymptomatic a Gram stain is not recommended because of lower sensitivity in this patient group.[23]
Laboratory evaluation: women
Performed by NAAT using self-collected vaginal swab or urine sample, or using clinician-collected endocervical swab.[23] Do not routinely collect urine samples if vaginal swab collection is possible because this provides the optimal specimen for NAATs to detect N gonorrhoeae.[23][48][56] Evidence suggests that the sensitivity and specificity of self-collected vaginal sampling are comparable to provider-collected sampling.[52] For determination of antimicrobial susceptibility, an endocervical swab is taken and sent for culture. A presumptive identification of N gonorrhoeae isolates recovered on selective medium can be made with a Gram stain and oxidase test. A presumptive identification indicates only that a gram-negative, oxidase-positive diplococcus is present; however, this is sufficient to start antimicrobial treatment. Additional tests are performed to confirm the identity of the isolate as N gonorrhoeae.
Women with vaginitis symptoms who do not have a cervix can have vaginal/urine culture or NAAT.
Laboratory evaluation: nongenital sites
Rectal and pharyngeal gonorrhea are often asymptomatic. Men who have sex with men (MSM) have been found to have a high incidence of pharyngeal gonorrhea[57][58] and rectal gonorrhea.[59] Routine screening of MSM at nongenital sites is advisable where possible. Screening high-risk women for rectal gonorrhea may identify cases that are not identifiable from cervical testing, even in the absence of a history of anal sex.[60] However, there are no recommendations for routine testing of the rectum in women.
NAATs are preferred to culture, and can be performed if using an FDA-approved system or the local laboratory is CLIA-compliant and authorized to carry out NAATs on nongenital specimens.[23][47][50]
Gram stains of endocervical, pharyngeal, or rectal specimens are not sufficient to detect infection and are therefore not recommended.[23][47]
Laboratory evaluation: patients with suspected DGI
Cultures need to be drawn from any sterile site that may be involved (e.g., blood, pharynx synovial fluid, or CSF).
Laboratory evaluation: infants with suspected gonococcal infection
In all cases of neonatal conjunctivitis, conjunctival exudates should be cultured for N gonorrhoeae and tested for antibiotic susceptibility before a definitive diagnosis is made.
Detection of gonococcal infection in neonates other than ophthalmia neonatorum requires cultures of the relevant body fluid or site. Positive gram-stained smears provide a presumptive basis for initiating treatment. However, diagnoses based on gram-stained smears or presumptive identification of cultures should be confirmed with definitive tests on culture isolates.[23]
Laboratory evaluation for suspected gonococcal infection: children, adolescents, and adults with possible sexual assault
NAATs can be used to detect N gonorrhoeae infection in a child, adolescent, or adult who might have been sexually assaulted. Specimens should be taken from sites of penetration or attempted penetration.[23]
Further investigations
If PID is suspected, a transvaginal ultrasound, computed tomography, or magnetic resonance imaging can be performed due to their high specificity for PID.
Use of this content is subject to our disclaimer