The evaluation of infertility focuses on identifying specific pathophysiologies. Female etiologies reported by women undergoing assisted reproductive technology (ART) procedures include tubal factor (10.4%), ovulatory dysfunction (14.3%), diminished ovarian reserve (26.9%), endometriosis (6.5%), and uterine factors (5.8%). Male etiologies are reported in 27.8% of ART cycles.[10]Centers for Disease Control and Prevention. 2021 assisted reproductive technology fertility clinic and national summary report. Atlanta, GA: US Dept of Health and Human Services; 2023.
https://www.cdc.gov/art/reports/2021/index.html
Male evaluation with at least a semen analysis is necessary while evaluating the female partner.
History
A complete medical and social history can reveal many infertility risk factors and provide focus for the remainder of the diagnostic workup. Pointers for female infertility include: age >35 years; irregular or no menses; history of sexually transmitted disease or other inflammatory pelvic process, including previous surgery (associated with tubal dysfunction); pelvic pain, including dyspareunia (associated with endometriosis or adenomyosis); very high or low body fat (linked to ovulatory disorders); or cigarette use (which may accelerate menopause). Occupational or other chemical exposure should also be considered.[63]Meyer JD, McDiarmid M, Diaz JH, et al. Reproductive and developmental hazard management. J Occup Environ Med. 2016 Mar;58(3):e94-e102.
https://journals.lww.com/joem/Fulltext/2016/03000/Reproductive_and_Developmental_Hazard_Management.23.aspx
http://www.ncbi.nlm.nih.gov/pubmed/26949895?tool=bestpractice.com
It is important that the history includes a thorough review of past medical illnesses (particularly childhood illnesses) that may have had an impact on ovarian function, including autoimmune disease (e.g., systemic lupus erythematosus, inflammatory bowel disease), obesity, or cancer.[48]Khizroeva J, Nalli C, Bitsadze V, et al. Infertility in women with systemic autoimmune diseases. Best Pract Res Clin Endocrinol Metab. 2019 Dec;33(6):101369.
http://www.ncbi.nlm.nih.gov/pubmed/31837981?tool=bestpractice.com
[77]Pourghazi F, Eslami M, Mohammadi S, et al. Association between childhood obesity and infertility in later life: a systematic review of cohort studies. BMC Endocr Disord. 2023 Oct 24;23(1):235.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10594820
http://www.ncbi.nlm.nih.gov/pubmed/37875830?tool=bestpractice.com
[78]van Santen HM, van de Wetering MD, Bos AME, et al. Reproductive complications in childhood cancer survivors. Pediatr Clin North Am. 2020 Dec;67(6):1187-202.
https://www.sciencedirect.com/science/article/pii/S0031395520301115
http://www.ncbi.nlm.nih.gov/pubmed/33131541?tool=bestpractice.com
Chemotherapy or pelvic radiation therapy for cancer can cause iatrogenic primary ovarian insufficiency.[79]Stuenkel CA, Gompel A. Primary ovarian insufficiency. N Engl J Med. 2023 Jan 12;388(2):154-63.
http://www.ncbi.nlm.nih.gov/pubmed/36630623?tool=bestpractice.com
Psychiatric history is also important as several dopaminergic medications used to treat psychiatric diseases can suppress hypothalamic-pituitary function and increase prolactin.[52]Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007 Oct;3(5):929-51.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2376090
http://www.ncbi.nlm.nih.gov/pubmed/18473017?tool=bestpractice.com
Women seeking fertility treatment may have a higher prevalence of current or past eating disorders, which can impact fertility and subsequent pregnancy outcomes.[53]Hecht LM, Hadwiger A, Patel S, et al. Disordered eating and eating disorders among women seeking fertility treatment: a systematic review. Arch Womens Ment Health. 2022 Feb;25(1):21-32.
http://www.ncbi.nlm.nih.gov/pubmed/34175997?tool=bestpractice.com
Surgical history may reveal risk factors for tubal disease, particularly pelvic surgery or appendectomy. Social history should include past history of sexually transmitted infection, past and current substance misuse, and the frequency and timing of intercourse.
Menstrual history is the best evidence of normal ovulatory function.[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
A normal menstrual cycle ranges from 21-35 days in length and menstrual bleeding occurs for 3-7 days. In a woman younger than 35 years of age, a history of a regular menstrual cycle is highly correlated with the presence of ovulation. This association is strengthened when menses are accompanied by monthly moliminal symptoms including breast tenderness, bloating, and/or mood changes. A long cycle is often associated with anovulation. By contrast, a short cycle may be associated with anovulation, inadequate follicular phase leading to poor endometrial development, or luteal phase deficiency. Some evidence suggests that a short cycle within the normal range (21-27 days) may be a sign of ovarian aging.[81]Younis JS, Iskander R, Fauser BCJM, et al. Does an association exist between menstrual cycle length within the normal range and ovarian reserve biomarkers during the reproductive years? A systematic review and meta-analysis. Hum Reprod Update. 2020 Nov 1;26(6):904-28.
https://academic.oup.com/humupd/article/26/6/904/5854778
http://www.ncbi.nlm.nih.gov/pubmed/32514566?tool=bestpractice.com
Dyspareunia, due to restricted uterus movement from peri-uterine adhesions, may be a presenting symptom suggesting tubal disease, endometriosis, or other anatomic causes of infertility.
Physical exam
Presenting signs may include very high or very low body fat (as indicated by body mass index, suggesting possible ovulatory dysfunction), galactorrhea, hirsutism, and/or acne. Pelvic examination may reveal abnormal shape and mobility of the uterus, the presence of nodularity in the cul de sac (suggesting endometriosis), and/or the presence of adnexal masses or tenderness.
Assessment of ovulation
The first step is to establish whether the woman is ovulating. A history of monthly menstrual cycles with premenstrual symptoms is indicative of ovulation. Women with regular cycles do not routinely require additional tests to confirm ovulation, but they may be considered in women with hirsutism or when menstrual history is indeterminate.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
Luteal-phase progesterone is a retrospective test of ovulation. Serum is assessed 7 days after the presumed day of ovulation (or 7 days before the presumed menstrual cycle). A progesterone value >3 ng/mL is indicative of an ovulatory cycle. The timing of this test is critical.[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
The use of peak values has low sensitivity and specificity. However, any elevation of progesterone concentration in the luteal phase is suggestive of ovulation, and thus further sampling (earlier in a shorter cycle, through to menses in a longer cycle) is often done, if the first value is indeterminate. Demonstration of an elevation in serum progesterone is considered confirmatory.
Ovulation can be detected prospectively and accurately with urinary luteinizing hormone (LH) prediction kits. This test uses an enzyme-linked immunoassay against the beta subunit of LH. LH rises abruptly for approximately 18 hours before it peaks, and ovulation typically occurs about 36 hours after the onset of the surge. Because the hormone needs to be conjugated before it is excreted, urinary LH will predict ovulation approximately 24 hours in advance. These tests are more accurate at predicting/demonstrating ovulation than basal body temperature charting, calendar calculation, or observation of vaginal or cervical discharge changes.[82]Eichener SF, Timpe EM. Urinary-based ovulation and pregnancy: point-of-care testing. Ann Pharmacother. 2004 Feb;38(2):325-31.
http://www.ncbi.nlm.nih.gov/pubmed/14742773?tool=bestpractice.com
Additionally, this provides a prospective assay of ovulation that also can be used to time intercourse.
Basal body temperature has been historically used as a means of determining ovulation because progesterone production from the corpus luteum raises core body temperature by approximately 0.6°F (0.3°C) providing a "biphasic" pattern of temperature. While couples may have already undertaken such assessment at home, it is not a reliable test of ovulation and is cumbersome to undertake effectively. Since there are better assessments of ovulation, it is no longer recommended and couples can be discouraged from persisting with it.
Serial ultrasound can be used to document follicular growth and ovulation.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
However, because serial measurements are required, this is expensive and time prohibitive as an initial diagnostic tool. It is commonly used by physicians to assess ovulation in patients who are taking infertility medications while in a treatment cycle.
Endometrial biopsy cannot discriminate between fertile and infertile women and should not be a routine part of the infertility evaluation.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
[83]Coutifaris C, Myers ER, Guzick DS, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004 Nov;82(5):1264-72.
http://www.ncbi.nlm.nih.gov/pubmed/15533340?tool=bestpractice.com
If ovulation is not confirmed, further diagnostic tests are indicated to establish the cause. These include assessment of follicle-stimulating hormone (FSH) and LH (hypergonadotropic or hypogonadotropic hypogonadism), estrogen levels, free testosterone (polycystic ovary syndrome or other causes of hyperandrogenism), serum thyroid-stimulating hormone (thyroid dysfunction), testing for celiac disease, prolactin (pituitary tumor), possible karyotype in the case of elevated gonadotropins (Turner syndrome) or recurrent pregnancy loss (Robertsonian translocation), and other secondary investigations to elucidate a cause. However, do not perform prolactin, immunologic, or thrombophilia testing as part of the routine infertility evaluation in women with regular menses.[1]National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. Sep 2017 [internet publication].
https://www.nice.org.uk/guidance/cg156
[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
[84]American Society for Reproductive Medicine. Ten things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2022 [internet publication].
https://web.archive.org/web/20221205142039/https://www.choosingwisely.org/societies/american-society-for-reproductive-medicine
Anatomic assessment
As an extension to physical exam, a transvaginal ultrasound scan (TVUS/S) is often undertaken in the clinic setting. A TVUS/S enables the assessment of pelvic organs, including the ovaries, for evidence of follicular development, antral follicle count, polycystic appearance, or the presence of significant cysts including endometriomas. It also enables the assessment of ovarian mobility/accessibility for oocyte retrieval if ultimately indicated. ATVUS/S also enables the assessment of the uterine structure (although this may require further clarification), including congenital abnormalities (such as uterine septum), the presence of fibroids, adenomyosis, and endometrial polyps. Sizeable hydrosalpinges, indicating tubal pathology, can also be detected. Thus, a TVUS/S informs the decision for further detailed pelvic assessment.
Assessment of the structural integrity of the reproductive tract is essential to the evaluation of fertility and necessary for all individuals. This can consist of radiologic imaging or surgical evaluation.
Historically, magnetic resonance imaging (MRI) was the preferred method of assessment for uterine abnormalities, predominantly Müllerian duct anomalies. MRI is reported to have high diagnostic accuracy for visualizing uterine abnormalities (100% specificity and 80% to 100% sensitivity for the evaluation of pelvic anomalies).[85]Pellerito JS, McCarthy SM, Doyle MB, et al. Diagnosis of uterine anomalies: relative accuracy of MR imaging, endovaginal sonography, and hysterosalpingography. Radiology. 1992 Jun;183(3):795-800.
http://www.ncbi.nlm.nih.gov/pubmed/1584936?tool=bestpractice.com
[86]Olpin JD, Heilbrun M. Imaging of Mullerian duct anomalies. Clin Obstet Gynecol. 2009 Mar;52(1):40-56.
http://www.ncbi.nlm.nih.gov/pubmed/19179860?tool=bestpractice.com
The production of images in multiple planes makes MRI an excellent preoperative assessment before reproductive gynecologic surgeries such as myomectomy or metroplasty. More recently, three-dimensional (3D) ultrasound has emerged as the imaging tool of choice for the assessment of the female pelvis. A 3D ultrasound scan enables a more detailed evaluation of the uterus, with reconstruction of anatomical planes, elusive to conventional TVUS/S.[87]Saravelos SH, Jayaprakasan K, Ojha K, et al. Assessment of the uterus with three-dimensional ultrasound in women undergoing ART. Hum Reprod Update. 2017 Mar 1;23(2):188-210.
https://academic.oup.com/humupd/article/23/2/188/2731727?login=false
http://www.ncbi.nlm.nih.gov/pubmed/28007752?tool=bestpractice.com
Uterine assessment with 3D ultrasound is cost effective, noninvasive and highly acceptable to women. Furthermore, it also has equivalent, if not higher, diagnostic accuracy to MRI, especially if used by experienced clinicians.[88]Ergenoglu AM, Sahin Ç, Şimşek D, et al. Comparison of three-dimensional ultrasound and magnetic resonance imaging diagnosis in surgically proven Müllerian duct anomaly cases. Eur J Obstet Gynecol Reprod Biol. 2016 Feb;197:22-6.
http://www.ncbi.nlm.nih.gov/pubmed/26699099?tool=bestpractice.com
The assessment of tubal patency is commonly undertaken in an outpatient setting. Chlamydia antibody testing may be considered as an initial, noninvasive test to assess the risk for tubal occlusion.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
A negative result suggests a low risk for tubal occlusion, but it does not rule out occlusion due to other infections. A positive result should be followed by further evaluation of tubal patency.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
Women who are not at risk of tubal obstruction because of comorbidities such as pelvic inflammatory disease, a previous ectopic pregnancy or endometriosis can be offered hysterosalpingography (HSG); those with comorbidities should be offered laparoscopy with chromopertubation or hysteroscopy.[1]National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. Sep 2017 [internet publication].
https://www.nice.org.uk/guidance/cg156
[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
HSG is performed by injecting radio-opaque dye into the uterus and following the dye with fluoroscopy. Uterine abnormalities are outlined by the dye, and tubal obstruction is noted by the absence of free-spill into the peritoneal cavity. According to one individual patient data meta-analysis, HSG has a pooled sensitivity of 53% and a pooled specificity of 87% for identification of any tubal pathology.[89]Broeze KA, Opmeer BC, Van Geloven N, et al. Are patient characteristics associated with the accuracy of hysterosalpingography in diagnosing tubal pathology? An individual patient data meta-analysis. Hum Reprod Update. 2011 May-Jun;17(3):293-300.
http://www.ncbi.nlm.nih.gov/pubmed/21147835?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Normal hysterosalpingography (HSG)From the collection of Dr Jared C. Robins [Citation ends].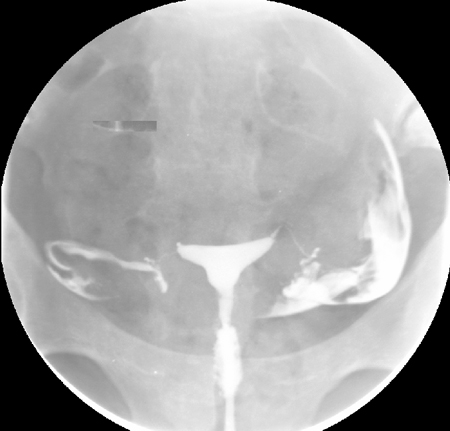 [Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends].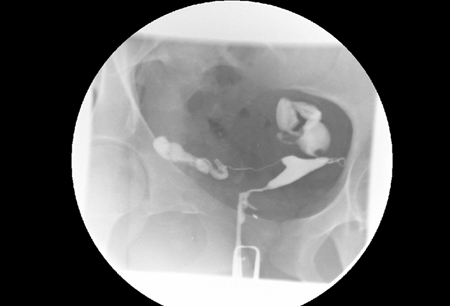
Because the positive predictive value of HSG is low, results suggesting tubal obstruction might require further evaluation.[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
A similar screening technique, hysterosalpingo-contrast sonography (HyCosY), uses TVUS in combination with a reflective medium injected transcervically to give a view of the endometrial cavity, as well as an assessment of tubal integrity.[90]Saunders RD, Shwayder JM, Nakajima ST. Current methods of tubal patency assessment. Fertil Steril. 2011 Jun;95(7):2171-9.
http://www.ncbi.nlm.nih.gov/pubmed/21457959?tool=bestpractice.com
HyCosY has a pooled sensitivity of 93% (95% CI 90% to 95%) and a pooled specificity of 90% (95% CI 87% to 92%) for the assessment of tubal patency.[91]Qu E, Zhang M, Ju J, et al. Is hysterosalpingo-contrast sonography (HyCoSy) using sulfur hexafluoride microbubbles (SonoVue) sufficient for the assessment of fallopian tube patency? A systematic review and meta-analysis. J Ultrasound Med. 2023 Jan;42(1):7-15.
http://www.ncbi.nlm.nih.gov/pubmed/35441714?tool=bestpractice.com
Although this imaging modality is more reliant on trained technicians, it has the advantage of avoiding radiation exposure, thereby gaining preference over HSG. Use of an oil-soluble contrast media for tubal flushing in the assessment of tubal patency may increase clinical pregnancy rates, and it is recommended over water-soluble contrast media.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
[92]Glanville EJ, Venetis C, Boothroyd CA, et al. The use of oil-soluble contrast media for tubal flushing in infertility: a consensus statement from ACCEPT (Australasian CREI Consensus Expert Panel on Trial evidence). Aust N Z J Obstet Gynaecol. 2020 Oct;60(5):667-70.
https://obgyn.onlinelibrary.wiley.com/doi/10.1111/ajo.13222
http://www.ncbi.nlm.nih.gov/pubmed/32776327?tool=bestpractice.com
[93]Wang R, Watson A, Johnson N, et al. Tubal flushing for subfertility. Cochrane Database Syst Rev. 2020 Oct 15;(10):CD003718.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003718.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/33053612?tool=bestpractice.com
The most common complication of oil-soluble contrast HSG is intravasation.[94]Roest I, Rosielle K, van Welie N, et al. Safety of oil-based contrast medium for hysterosalpingography: a systematic review. Reprod Biomed Online. 2021 Jun;42(6):1119-29.
https://www.rbmojournal.com/article/S1472-6483(21)00142-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33931367?tool=bestpractice.com
Oil embolism is a rare complication, but the technique should be performed with fluorescence guidance.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
Saline-infusion sonography (SIS) can be used to follow up intrauterine abnormalities seen on HSG or evaluate the uterus if there is no suspicion about the fallopian tubes. Traditional ultrasonography is not sensitive enough to determine whether lesions are intracavitary, because the uterus is a potential space. Injecting saline into the uterus to provide a sonographic window within the endometrial cavity enables better visualization. The sensitivity and specificity of SIS were both estimated to be 100% when surgery was used as a definitive test.[95]Sylvestre C, Child TJ, Tulandi T, et al. A prospective study to evaluate the efficacy of two- and three-dimensional sonohysterography in women with intrauterine lesions. Fertil Steril. 2003 May;79(5):1222-5.
http://www.ncbi.nlm.nih.gov/pubmed/12738522?tool=bestpractice.com
[96]Nieuwenhuis LL, Hermans FJ, Bij de Vaate AJM, et al. Three-dimensional saline infusion sonography compared to two-dimensional saline infusion sonography for the diagnosis of focal intracavitary lesions. Cochrane Database Syst Rev. 2017 May 5;5:CD011126.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011126.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28472862?tool=bestpractice.com
Although radiologic imaging provides information about the viscous portions of pelvic structures, it provides little information about peritubal adhesions or endometriosis. Furthermore, patients with risk factors, such as previous pelvic infections, are not suitable candidates for invasive imaging in an office setting. Laparoscopy is the gold standard investigation to further this diagnostic workup. However, this would be in specific circumstances. Do not perform laparoscopy routinely for the evaluation of unexplained infertility.[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
[84]American Society for Reproductive Medicine. Ten things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2022 [internet publication].
https://web.archive.org/web/20221205142039/https://www.choosingwisely.org/societies/american-society-for-reproductive-medicine
The decision to perform laparoscopy as an initial diagnostic modality is based on clinical suspicion. For example, in a woman with a history of cyclic pelvic pain suggestive of endometriosis, laparoscopy may be the best initial evaluation. In selected cases, hysteroscopy at the time of laparoscopy may be indicated (e.g., to clarify uterine abnormalities or assess submucous fibroids). Hysteroscopy has a pooled sensitivity and specificity of 88% and 85%, respectively, for detection of tubal obstruction.[97]Vitale SG, Carugno J, Riemma G, et al. Hysteroscopy for assessing fallopian tubal obstruction: a systematic review and diagnostic test accuracy meta-analysis. J Minim Invasive Gynecol. 2021 Apr;28(4):769-78.
http://www.ncbi.nlm.nih.gov/pubmed/33246040?tool=bestpractice.com
In addition to direct visualization for diagnostic purposes, laparoscopy and hysteroscopy may also confer the benefit of surgical treatment at the same time. Some evidence suggests that hysteroscopy may improve live birth rates in women undergoing assisted reproductive technology, even when initial imaging is normal.[98]Hou JH, Lu BJ, Huang YL, et al. Outpatient hysteroscopy impact on subsequent assisted reproductive technology: a systematic review and meta-analysis in patients with normal transvaginal sonography or hysterosalpingography images. Reprod Biol Endocrinol. 2024 Feb 1;22(1):18.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10832084
http://www.ncbi.nlm.nih.gov/pubmed/38302947?tool=bestpractice.com
[99]Vitale SG, Angioni S, Parry JP, et al. Efficacy of hysteroscopy in improving fertility outcomes in women undergoing assisted reproductive technique: a systematic review and meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2023;88(6):336-48.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10794974
http://www.ncbi.nlm.nih.gov/pubmed/37899034?tool=bestpractice.com
However, one Cochrane review noted that results were inconclusive when only considering trials at low risk of bias, and guidelines recommend against hysteroscopy when imaging is normal.[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
[100]Kamath MS, Bosteels J, D'Hooghe TM, et al. Screening hysteroscopy in subfertile women and women undergoing assisted reproduction. Cochrane Database Syst Rev. 2019 Apr 16;4(4):CD012856.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012856.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/30991443?tool=bestpractice.com
[  ]
For women undergoing in vitro fertilization (IVF), does prior screening hysteroscopy help to improve outcomes?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2595/fullShow me the answer[Evidence C]b2ca60f2-9ea2-470a-aa83-beabf34d0d8bccaCFor women undergoing in vitro fertilization (IVF), does prior screening hysteroscopy help to improve outcomes?
]
For women undergoing in vitro fertilization (IVF), does prior screening hysteroscopy help to improve outcomes?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2595/fullShow me the answer[Evidence C]b2ca60f2-9ea2-470a-aa83-beabf34d0d8bccaCFor women undergoing in vitro fertilization (IVF), does prior screening hysteroscopy help to improve outcomes?
Do not perform a postcoital test to assess the cervix and cervical mucus as part of the evaluation for infertility. There is high intra-observer variation in this test, and it is no longer recommended for routine use.[1]National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. Sep 2017 [internet publication].
https://www.nice.org.uk/guidance/cg156
[2]European Society of Human Reproduction and Embryology. Unexplained infertility: guideline of European Society of Human Reproduction and Embryology. 2023 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Unexplained-infertility
[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
[84]American Society for Reproductive Medicine. Ten things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2022 [internet publication].
https://web.archive.org/web/20221205142039/https://www.choosingwisely.org/societies/american-society-for-reproductive-medicine
[Figure caption and citation for the preceding image starts]: Normal hysterosalpingography (HSG)From the collection of Dr Jared C. Robins [Citation ends]. [Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends]. [Figure caption and citation for the preceding image starts]: Normal saline infusion ultrasoundFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Normal saline infusion ultrasoundFrom the collection of Dr Jared C. Robins [Citation ends].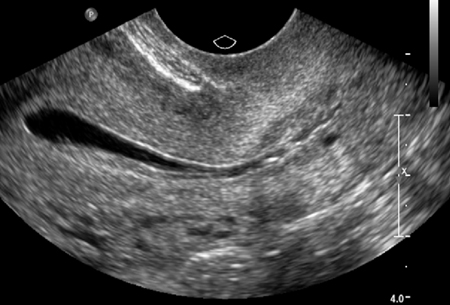 [Figure caption and citation for the preceding image starts]: Saline infusion ultrasound with polypFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Saline infusion ultrasound with polypFrom the collection of Dr Jared C. Robins [Citation ends].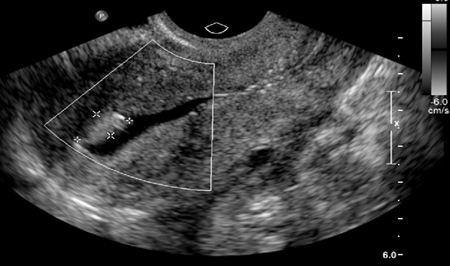
Ovarian reserve testing
Age is the best predictor of fertility.[12]American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014 Mar;101(3):633-4.
https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/03/female-age-related-fertility-decline
http://www.ncbi.nlm.nih.gov/pubmed/24559617?tool=bestpractice.com
[13]American College of Obstetricians and Gynecologists’ Committee on Clinical Consensus-Obstetrics; Gantt A; Society for Maternal-Fetal Medicine; et al. Obstetric Care Consensus #11, Pregnancy at age 35 years or older. Am J Obstet Gynecol. 2023 Mar;228(3):B25-40.
https://www.acog.org/clinical/clinical-guidance/obstetric-care-consensus/articles/2022/08/pregnancy-at-age-35-years-or-older
http://www.ncbi.nlm.nih.gov/pubmed/35850202?tool=bestpractice.com
Many researchers have sought to establish assays of "ovarian aging" to assess the quality and quantity of the remaining oocytes in an attempt to predict reproductive potential.[101]Domingues TS, Rocha AM, Serafini PC. Tests for ovarian reserve: reliability and utility. Curr Opinion Obstet Gynecol. 2010 Aug;22(4):271-6.
http://www.ncbi.nlm.nih.gov/pubmed/20543692?tool=bestpractice.com
The most commonly used tests are the measurement of basal FSH on day 2-5 of the menstrual cycle (levels >10 IU/L may be considered consistent with diminished ovarian reserve and levels >8.9 IU/L may be considered predictive of a low response to future ovarian stimulation), a total antral follicle count undertaken by TVUS on day 3 of the menstrual cycle (<5-7 oocytes may be considered consistent with diminished ovarian reserve and ≤4 oocytes may be considered predictive of a low response to future ovarian stimulation), and measurement of anti-Müllerian hormone (AMH), which is not cycle-day specific (<1 ng/mL [<7.1 pmol/L] may be considered consistent with diminished ovarian reserve and ≤0.76 ng/mL [≤5.4 pmol/L] may be considered predictive of a low response to future ovarian stimulation).[1]National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. Sep 2017 [internet publication].
https://www.nice.org.uk/guidance/cg156
[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
[102]La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010 Mar-Apr;16(2):113-30.
https://academic.oup.com/humupd/article/16/2/113/738211
http://www.ncbi.nlm.nih.gov/pubmed/19793843?tool=bestpractice.com
[  ]
How do ovarian reserve test algorithms for gonadotropin dose selection compare with standard dose for women undergoing in vitro fertilization plus intracytoplasmic sperm injection?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2028/fullShow me the answer
]
How do ovarian reserve test algorithms for gonadotropin dose selection compare with standard dose for women undergoing in vitro fertilization plus intracytoplasmic sperm injection?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2028/fullShow me the answer
Basal FSH and estradiol can be measured together in the early follicular phase to assess ovarian reserve; however, estradiol (E2) should not be measured in isolation to predict the outcome of fertility treatment.[1]National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. Sep 2017 [internet publication].
https://www.nice.org.uk/guidance/cg156
[80]Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021 Nov;116(5):1255-65.
https://www.asrm.org/globalassets/_asrm/practice-guidance/practice-guidelines/pdf/diagnostic_evaluation_of_the_infertile_female.pdf
http://www.ncbi.nlm.nih.gov/pubmed/34607703?tool=bestpractice.com
Elevated estradiol levels may suppress FSH levels, so the value of measuring estradiol levels is that they provide context for normal FSH levels. High estradiol levels are, therefore, associated with reproductive aging and diminished ovarian reserve.
Most studies suggest that abnormal basal hormonal levels are predictive of response to fertility medication, but not highly predictive of pregnancy or failure to conceive, especially in young women.[103]Maheshwari A, Bhattacharya S, Johnson NP. Predicting fertility. Hum Fertil (Camb). 2008 Jun;11(2):109-17.
http://www.ncbi.nlm.nih.gov/pubmed/18569066?tool=bestpractice.com
So-called dynamic ovarian reserve testing (e.g., clomiphene challenge) confers little additional benefit.[104]Broekmans FJ, Kwee J, Hendriks DJ, et al. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006 Nov-Dec;12(6):685-718.
http://humupd.oxfordjournals.org/content/12/6/685.long
http://www.ncbi.nlm.nih.gov/pubmed/16891297?tool=bestpractice.com
Ovarian reserve testing may be useful for patients with unexplained infertility or for those at risk of ovarian failure. Examples of risks for ovarian failure include age older than 35 years, family history of primary ovarian insufficiency, history of ovarian surgery, or smoking.
 [Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends].
 ]
[Evidence C]
]
[Evidence C] [Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Hysterosalpingography (HSG) demonstrating bilateral hydrosalpingesFrom the collection of Dr Jared C. Robins [Citation ends]. [Figure caption and citation for the preceding image starts]: Normal saline infusion ultrasoundFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Normal saline infusion ultrasoundFrom the collection of Dr Jared C. Robins [Citation ends]. [Figure caption and citation for the preceding image starts]: Saline infusion ultrasound with polypFrom the collection of Dr Jared C. Robins [Citation ends].
[Figure caption and citation for the preceding image starts]: Saline infusion ultrasound with polypFrom the collection of Dr Jared C. Robins [Citation ends].
 ]
]