Etiology
The presence of persistently elevated antiphospholipid antibodies (aPL) to various phospholipid-binding plasma proteins can result in the development of venous, arterial, and microvascular thromboses and/or pregnancy-associated morbidity. It is unclear what leads to the development of these acquired antibodies; it may be that molecular mimicry occurs through exposure to a viral or bacterial infection.[Figure caption and citation for the preceding image starts]: Fibrin immunostain of renal biopsy demonstrating features consistent with thrombotic microangiopathyFrom the personal teaching collection of Professor Hunt; used with permission [Citation ends].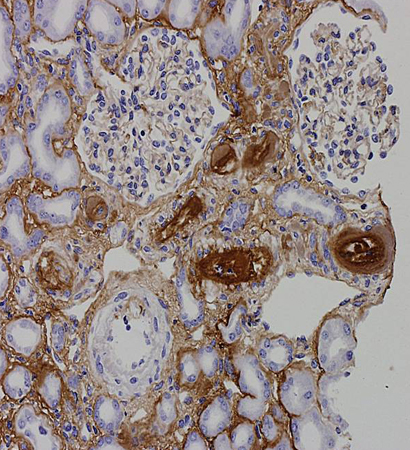 [Figure caption and citation for the preceding image starts]: Trichrome stain of renal biopsy demonstrating features consistent with thrombotic microangiopathyFrom the personal teaching collection of Professor Hunt; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Trichrome stain of renal biopsy demonstrating features consistent with thrombotic microangiopathyFrom the personal teaching collection of Professor Hunt; used with permission [Citation ends].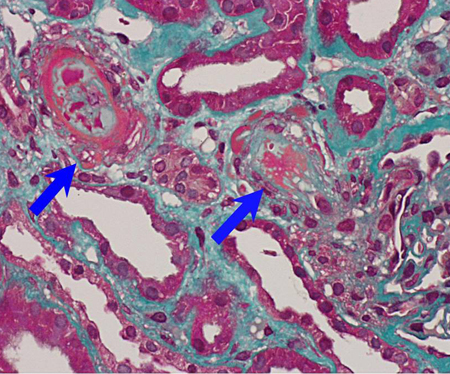
Pathophysiology
aPL are autoantibodies that recognize a variety of phospholipid-binding plasma proteins such as beta2-glycoprotein I, prothrombin, annexin A5, and phospholipids expressed on platelets, monocytes, and trophoblast cells. Several mechanisms have been proposed to describe antiphospholipid antibody-mediated thrombosis and pregnancy morbidity, but there is no widely accepted unifying mechanism to describe all the phenomena of the disease.[13][14][15] Beta2-glycoprotein I, a complement control protein constructed of five domains, is recognized as the major antigen target of APS antibodies. Antibodies recognizing a particular epitope in domain I of beta2-glycoprotein I have been found to be associated with an increased risk of thrombosis.[16]
Other mechanisms proposed for the role of APS antibodies in thrombosis include endothelial binding, protein C resistance, and complement activation.[17][18][19][20] Several thrombotic and inflammatory-mediated mechanisms induced by the binding of aPL may underlie the pathogenesis of antiphospholipid antibodies in pregnancy-related complications.[21] In particular, the complement system has been implicated in the pathogenesis of thrombosis and fetal loss in APS in mice, and resistance to annexin A5 may also be a significant mechanism.[22][23][24][25][26]
Classification
International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS)[1]
There are no diagnostic criteria for APS. However, the classification criteria (originally formulated in Sapporo, Japan, for research purposes) is often used as a guide for APS diagnosis.[1][3] The Sapporo criteria have a specificity of 86% and a sensitivity of 99%.[1] APS is diagnosed if at least one of the clinical criteria of vascular thrombosis or pregnancy morbidity is present in association with the presence of antiphospholipid antibodies on two or more occasions, 12 weeks apart.[1] Vascular thrombosis is defined as one or more clinical episodes of arterial, venous, or small-vessel thrombosis, in any tissue or organ.
Pregnancy morbidity is defined as the loss of three or more embryos before the 10th week of gestation and/or one or more otherwise unexplained fetal deaths between the 10th week of gestation and the 34th week of gestation, and/or the premature birth of a morphologically normal neonate before the 34th week of gestation because of eclampsia, severe preeclampsia, intrauterine growth restriction, or placental insufficiency.[1]
American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) antiphospholipid syndrome classification criteria[2]
The ACR/EULAR classification criteria update the Sapporo criteria above, by widening the scope of symptoms and clinical signs to include hierarchically clustered and weighted independent clinical and laboratory domains.[2] The new criteria, designed to improve APS-related research, have a specificity of 99% and sensitivity of 84%.[2] Those with at least one positive aPL test within 3 years of an aPL-associated clinical criterion can be classified with the ACR/EULAR criteria. Clinical features are now divided into six domains (macrovascular venous thromboembolism, macrovascular arterial thrombosis, microvascular, obstetric, cardiac valve, and hematologic) and there are also two laboratory domains (aPL tests by coagulation assay and solid-phase assay). Items in each of these eight domains receive a score between 1 and 7. People who score at least 3 points in the clinical domains and at least 3 points in the laboratory domains are classified as having APS.[2] Similar to the Sapporo criteria, the ACR/EULAR classification is primarily intended to identify homogeneous groups with a high likelihood of APS for research purposes, rather than being diagnostic, but it is a useful guide for clinicians in the evaluation of aPL-positive individuals.
Use of this content is subject to our disclaimer