The diagnosis of Crohn disease (CD) is suggested by a typical history supported by physical findings, especially perianal involvement and radiologic and endoscopic investigations.
The clinical presentation of CD and the order of investigations performed varies based on the part of the gastrointestinal tract involved, the degree of inflammation, and presence of complications. The American Gastroenterological Association (AGA) suggests using a combination of biomarker-based plus symptom-based monitoring strategy over symptom-based strategy alone in patients with CD in symptomatic remission.[66]Ananthakrishnan AN, Adler J, Chachu KA, et al. AGA clinical practice guideline on the role of biomarkers for the management of crohn's disease. Gastroenterology. 2023 Dec;165(6):1367-99.
https://www.gastrojournal.org/article/S0016-5085(23)05064-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37981354?tool=bestpractice.com
History
A family history of inflammatory bowel disease (IBD) increases the likelihood of CD. Approximately 12% of patients have a family history of CD.[57]Santos MPC, Gomes C, Torres J. Familial and ethnic risk in inflammatory bowel disease. Ann Gastroenterol. 2018 Jan-Feb;31(1):14-23.
https://pmc.ncbi.nlm.nih.gov/articles/PMC5759609
http://www.ncbi.nlm.nih.gov/pubmed/29333063?tool=bestpractice.com
It is more common in white than black or Asian people, and in individuals of Ashkenazi Jewish origin.[11]Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017 Jul;92(7):1088-103.
https://www.mayoclinicproceedings.org/article/S0025-6196(17)30313-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28601423?tool=bestpractice.com
[19]Aniwan S, Harmsen WS, Tremaine WJ, et al. Incidence of inflammatory bowel disease by race and ethnicity in a population-based inception cohort from 1970 through 2010. Therap Adv Gastroenterol. 2019 Feb 6;12:1756284819827692.
https://journals.sagepub.com/doi/10.1177/1756284819827692
http://www.ncbi.nlm.nih.gov/pubmed/30792818?tool=bestpractice.com
[20]Betteridge JD, Armbruster SP, Maydonovitch C, et al. Inflammatory bowel disease prevalence by age, gender, race, and geographic location in the US military health care population. Inflamm Bowel Dis. 2013 Jun;19(7):1421-7.
http://www.ncbi.nlm.nih.gov/pubmed/23518811?tool=bestpractice.com
Ashkenazi Jews have a two- to fourfold increased risk of CD.[56]Kenny EE, Pe'er I, Karban A, et al. A genome-wide scan of Ashkenazi Jewish Crohn's disease suggests novel susceptibility loci. PLoS Genet. 2012;8(3):e1002559.
https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1002559
http://www.ncbi.nlm.nih.gov/pubmed/22412388?tool=bestpractice.com
The onset of CD typically occurs in the second to fourth decade of life with a smaller peak from 50-60 years.[11]Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017 Jul;92(7):1088-103.
https://www.mayoclinicproceedings.org/article/S0025-6196(17)30313-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28601423?tool=bestpractice.com
[12]Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet. 2017 Apr 29;389(10080):1741-55.
http://www.ncbi.nlm.nih.gov/pubmed/27914655?tool=bestpractice.com
[13]Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46-54;e42;quiz e30.
https://www.gastrojournal.org/article/S0016-5085(11)01378-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/22001864?tool=bestpractice.com
The clinical picture of CD includes different combinations of symptoms including fatigue, diarrhea, abdominal pain, weight loss, fever, and gastrointestinal bleeding.[9]Kalla R, Ventham NT, Satsangi J, et al. Crohn's disease. BMJ. 2014 Nov 19;349:g6670.[12]Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet. 2017 Apr 29;389(10080):1741-55.
http://www.ncbi.nlm.nih.gov/pubmed/27914655?tool=bestpractice.com
Diagnosis can be difficult in view of nonspecific symptoms.
Exclude alternative causes
The history should also exclude alternative causes for symptoms. Recent travel, recent antibiotic use, or contact with sick people suggest an infectious cause for diarrhea.
Intestinal tuberculosis (TB) is an important differential diagnosis that should not be missed.[67]Kedia S, Das P, Madhusudhan KS, et al. Differentiating Crohn's disease from intestinal tuberculosis. World J Gastroenterol. 2019 Jan 28;25(4):418-32.
https://www.wjgnet.com/1007-9327/full/v25/i4/418.htm
http://www.ncbi.nlm.nih.gov/pubmed/30700939?tool=bestpractice.com
Mistakenly treating patients for CD with immunosuppression could be life-threatening.[68]Gurzu S, Molnar C, Contac AO, et al. Tuberculosis terminal ileitis: a forgotten entity mimicking Crohn's disease. World J Clin Cases. 2016 Sep 16;4(9):273-80.
https://www.wjgnet.com/2307-8960/full/v4/i9/273.htm
http://www.ncbi.nlm.nih.gov/pubmed/27672643?tool=bestpractice.com
In cases of high suspicion, patients may require empiric treatment for TB under specialist guidance.[69]Ooi CJ, Makharia GK, Hilmi I, et al. Asia Pacific consensus statements on Crohn's disease. Part 1: definition, diagnosis, and epidemiology. J Gastroenterol Hepatol. 2016 Jan;31(1):45-55.
https://onlinelibrary.wiley.com/doi/full/10.1111/jgh.12956
http://www.ncbi.nlm.nih.gov/pubmed/25819140?tool=bestpractice.com
See Extrapulmonary tuberculosis.
Physical exam
Patients present with a constellation of abdominal findings including right lower quadrant abdominal tenderness, and palpable abdominal mass.
The following should be performed:
Oral inspection for ulcers
Abdominal exam for masses or pain
Perineal inspection for perianal skin tags, fistulae, abscesses, and sinus tracts
Digital rectal exam for occult blood and exclusion of a mass
The skin should be inspected for signs of extraintestinal skin manifestations of CD, such as erythema nodosum and pyoderma gangrenosum. Body mass index should be calculated as a baseline for future changes in weight.[Figure caption and citation for the preceding image starts]: A patient's arms and hands show the presence of erythema nodosumCDC/ Margaret Renz [Citation ends].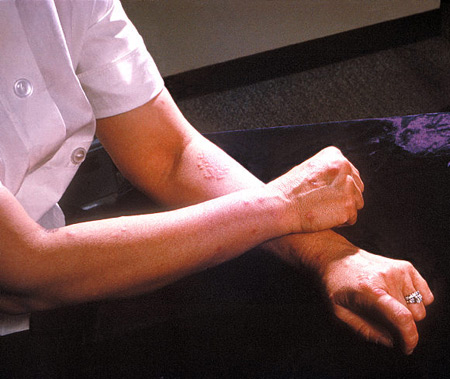
Initial laboratory investigations
All patients should have a complete blood count, comprehensive metabolic panel, C-reactive protein, and erythrocyte sedimentation rate on initial presentation.[9]Kalla R, Ventham NT, Satsangi J, et al. Crohn's disease. BMJ. 2014 Nov 19;349:g6670.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
Serum iron studies and vitamin B12 and folate levels should be performed.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[72]Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015 Mar;9(3):211-22.
https://academic.oup.com/ecco-jcc/article/9/3/211/361529
http://www.ncbi.nlm.nih.gov/pubmed/25518052?tool=bestpractice.com
Stool should be sent for microscopy (including for ova, cysts, and parasites) and culture.[9]Kalla R, Ventham NT, Satsangi J, et al. Crohn's disease. BMJ. 2014 Nov 19;349:g6670.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
Testing for Clostridium difficile toxin is indicated, especially if there is a history of recent antibiotic use.[9]Kalla R, Ventham NT, Satsangi J, et al. Crohn's disease. BMJ. 2014 Nov 19;349:g6670. C difficile infection is associated with increased short- and long-term mortality in patients with IBD.[73]Balram B, Battat R, Al-Khoury A, et al. Risk factors associated with Clostridium difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2019 Jan 1;13(1):27-38.
https://academic.oup.com/ecco-jcc/article/13/1/27/5104729
http://www.ncbi.nlm.nih.gov/pubmed/30247650?tool=bestpractice.com
Yersinia enterocolitica serology should be requested in patients with clinical suspicion of ileitis.[74]Matsumoto T, Iida M, Matsui T, et al. Endoscopic findings in Yersinia enterocolitica enterocolitis. Gastrointest Endosc. 1990 Nov-Dec;36(6):583-7.
http://www.ncbi.nlm.nih.gov/pubmed/2279647?tool=bestpractice.com
Imaging studies
Plain abdominal radiographs may be part of the initial tests ordered in the acute setting. They are not diagnostic of CD, but may be suggestive and help in assessing severity. Bowel loop distention and pneumoperitoneum may be visible.
Computed tomography (CT) and magnetic resonance (MR) studies
CT and MR studies offer higher-resolution imaging than contrast radiologic studies, as well as additional information on abdominal organs and structures (e.g., lymphadenopathy and malignancies) to aid diagnosis.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
Patients who are able to tolerate oral contrast are candidates for CT enterography or MR enterography.[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
CT enterography is sensitive (75% to 90%), and can assess for alternative diagnoses, and potential complications of CD (e.g., obstruction, abscess, fistula).[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
MR enterography characteristics (sensitivity 77% to 82%, specificity 80% to 100%) are similar to those of CT enterography, but availability may be limited.[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
MR imaging has also been shown to yield robust diagnostic accuracy in detecting fibrotic strictures, distinguishing between fibrotic and inflammatory strictures, and evaluating stricture severity in patients with CD.[76]Kobeissy A, Merza N, Nawras Y, et al. Evaluating the diagnostic accuracy of magnetic resonance imaging in distinguishing strictures in Crohn's disease: a systematic review and meta-analysis. Int J Colorectal Dis. 2023 Oct 26;38(1):258.
http://www.ncbi.nlm.nih.gov/pubmed/37882852?tool=bestpractice.com
Guidelines suggest that MR enterography should be the test of choice as it does not expose the patient to ionizing radiation.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[77]Bruining DH, Zimmermann EM, Loftus EV Jr, et al; Society of Abdominal Radiology Crohn’s Disease-Focused Panel. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn's disease. Radiology. 2018 Mar;286(3):776-99.
https://pubs.rsna.org/doi/10.1148/radiol.2018171737
http://www.ncbi.nlm.nih.gov/pubmed/29319414?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: CT scan demonstrating thickening of the terminal ileum in a patient with Crohn disease exacerbationProvided by Drs Wissam Bleibel, Bishal Mainali, Chandrashekhar Thukral, and Mark A. Peppercorn, the previous authors of this topic [Citation ends].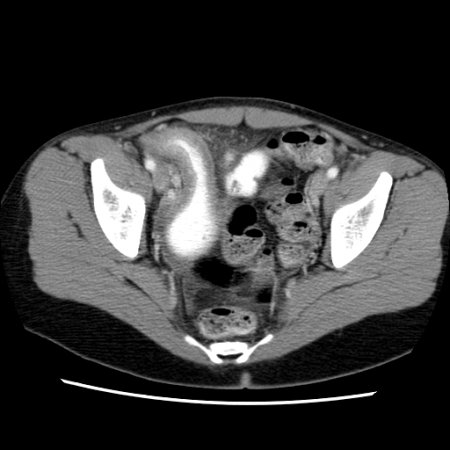 [Figure caption and citation for the preceding image starts]: CT scan demonstrating thickening of the terminal ileum in a patient with Crohn disease exacerbationProvided by Drs Wissam Bleibel, Bishal Mainali, Chandrashekhar Thukral, and Mark A. Peppercorn, the previous authors of this topic [Citation ends].
[Figure caption and citation for the preceding image starts]: CT scan demonstrating thickening of the terminal ileum in a patient with Crohn disease exacerbationProvided by Drs Wissam Bleibel, Bishal Mainali, Chandrashekhar Thukral, and Mark A. Peppercorn, the previous authors of this topic [Citation ends].
CT enterography and MR enterography are useful for postoperative assessment and evaluation of recurrence.[78]Yung DE, Har-Noy O, Tham YS, et al. Capsule endoscopy, magnetic resonance enterography, and small bowel ultrasound for evaluation of postoperative recurrence in Crohn's disease: systematic review and meta-analysis. Inflamm Bowel Dis. 2017 Dec 19;24(1):93-100.
https://academic.oup.com/ibdjournal/article/24/1/93/4757506
http://www.ncbi.nlm.nih.gov/pubmed/29272490?tool=bestpractice.com
If CT is indicated, do not order unenhanced CT alongside intravenous contrast-enhanced CT because the addition of unenhanced CT does not provide additional diagnostic information and exposes patients to unnecessary radiation.[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
[79]American College of Radiology. Ten things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2021 [internet publication].
https://web.archive.org/web/20230330210926/https://www.choosingwisely.org/societies/american-college-of-radiology
Ultrasonography
Ultrasound of the abdomen and pelvis may be considered.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
Sensitivity and specificity range from 75% to 94% and 67% to 100%, respectively.[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
Ultrasound may facilitate detection of extramural complications, detection and evaluation of stenotic strictures, and monitoring of disease course.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[80]Bots S, De Voogd F, De Jong M, et al. Point-of-care intestinal ultrasound in IBD patients: disease management and diagnostic yield in a real-world cohort and proposal of a point-of-care algorithm. J Crohns Colitis. 2022 May 10;16(4):606-15.
https://academic.oup.com/ecco-jcc/article/16/4/606/6391192?login=false
http://www.ncbi.nlm.nih.gov/pubmed/34636839?tool=bestpractice.com
Obesity, overlying bowel gas, and guarding (in the acutely unwell patient) may preclude adequate compression of the ultrasound probe.[81]Chavannes M, Dolinger MT, Cohen-Mekelburg S, et al. AGA clinical practice update on the role of intestinal ultrasound in inflammatory bowel disease: commentary. Clin Gastroenterol Hepatol. 2024 Sep;22(9):1790-5.e1.
https://www.cghjournal.org/article/S1542-3565(24)00454-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39001773?tool=bestpractice.com
An accuracy comparable to CT or MR modalities has been noted for visualization of ileum when intestinal ultrasound is performed by trained practitioners.[81]Chavannes M, Dolinger MT, Cohen-Mekelburg S, et al. AGA clinical practice update on the role of intestinal ultrasound in inflammatory bowel disease: commentary. Clin Gastroenterol Hepatol. 2024 Sep;22(9):1790-5.e1.
https://www.cghjournal.org/article/S1542-3565(24)00454-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39001773?tool=bestpractice.com
Limitations of this technique include limited visualization of the stomach, esophagus, and rectum and inability for performing interventional procedure.[81]Chavannes M, Dolinger MT, Cohen-Mekelburg S, et al. AGA clinical practice update on the role of intestinal ultrasound in inflammatory bowel disease: commentary. Clin Gastroenterol Hepatol. 2024 Sep;22(9):1790-5.e1.
https://www.cghjournal.org/article/S1542-3565(24)00454-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39001773?tool=bestpractice.com
Endoscopy
Ileocolonoscopy with biopsies should be performed in the assessment of suspected CD.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
Ileocolonoscopy and imaging studies are complementary in the diagnosis of CD.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[75]American College of Radiology. ACR appropriateness criteria: Crohn disease. 2019 [internet publication].
https://acsearch.acr.org/docs/69470/Narrative
Mucosal changes suggestive of CD include mucosal nodularity, erythema, edema, ulcerations, friability, stenosis, and the identification of fistulous tract opening.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
Lesions are discontinuous, with intermittent areas of normal-appearing bowel (skip lesions). Evidence of a normal rectum and isolated involvement of the terminal ileum support the diagnosis.[Figure caption and citation for the preceding image starts]: Endoscopic view of Crohn ileitisProvided by Drs Wissam Bleibel, Bishal Mainali, Chandrashekhar Thukral, and Mark A. Peppercorn, the previous authors of this topic [Citation ends].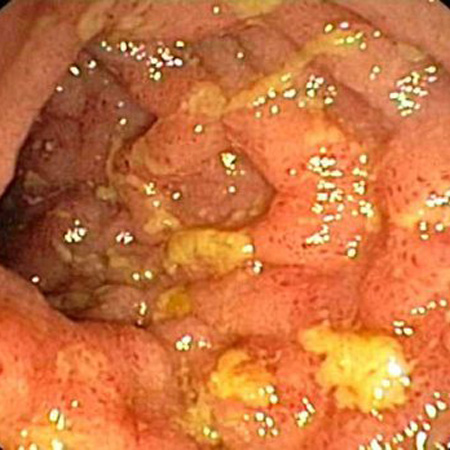
Segmental colonic and ileal biopsies should be obtained to assess for microscopic evidence of CD.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
The microscopic features that help distinguish ulcerative colitis and CD include granulomas, architectural change, and disease distribution.[82]Feakins RM; British Society of Gastroenterology. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013 Dec;66(12):1005-26.
http://jcp.bmj.com/content/66/12/1005.long
http://www.ncbi.nlm.nih.gov/pubmed/23999270?tool=bestpractice.com
Granulomatous inflammation is, however, reported in a minority of patients with CD (30% to 50%); it is not required for diagnosis.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[82]Feakins RM; British Society of Gastroenterology. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013 Dec;66(12):1005-26.
http://jcp.bmj.com/content/66/12/1005.long
http://www.ncbi.nlm.nih.gov/pubmed/23999270?tool=bestpractice.com
Biopsies of uninvolved mucosa help to identify the extent of histologic disease.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
If TB is being considered, tissue from ileocecal biopsies can be tested and cultured for Mycobacterium tuberculosis.[69]Ooi CJ, Makharia GK, Hilmi I, et al. Asia Pacific consensus statements on Crohn's disease. Part 1: definition, diagnosis, and epidemiology. J Gastroenterol Hepatol. 2016 Jan;31(1):45-55.
https://onlinelibrary.wiley.com/doi/full/10.1111/jgh.12956
http://www.ncbi.nlm.nih.gov/pubmed/25819140?tool=bestpractice.com
Esophagogastroduodenoscopy (upper gastrointestinal endoscopy) is not routinely required as part of the diagnostic evaluation, but may be performed in patients with upper gastrointestinal signs and symptoms.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
Capsule endoscopy can be used as an alternative to MR imaging to assess small bowel involvement. Patency capsule evaluation is advised prior to endoscopy to reduce the risk of capsule retention if there is a possibility of strictures.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
Fecal calprotectin
Fecal calprotectin is a noninvasive marker of bowel inflammation.[9]Kalla R, Ventham NT, Satsangi J, et al. Crohn's disease. BMJ. 2014 Nov 19;349:g6670.[83]van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010 Jul 15;341:c3369.
https://www.bmj.com/content/341/bmj.c3369
http://www.ncbi.nlm.nih.gov/pubmed/20634346?tool=bestpractice.com
[84]Kopylov U, Yung DE, Engel T, et al. Fecal calprotectin for the prediction of small-bowel Crohn's disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016 Oct;28(10):1137-44.
http://www.ncbi.nlm.nih.gov/pubmed/27415156?tool=bestpractice.com
It is released into feces when neutrophils gather at the site of any GI tract inflammation. In patients ages <60 years having lower GI symptoms and normal initial workup, fecal calprotectin testing can be performed to exclude causes of colonic inflammation. However, in patients ages >60 years with suspected colorectal cancer, fecal calprotectin testing should be interpreted with caution.
Fecal calprotectin can help to differentiate IBD from irritable bowel syndrome.[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
Fecal calprotectin is not specific for CD and can be increased in the stool in other gut pathologies (e.g., infectious gastroenteritis, diverticulitis, or colorectal cancer) or in patients on nonsteroidal anti-inflammatory drugs [NSAIDs] and aspirin).[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
[85]Deputy M, Devanaboina R, Al Bakir I, et al. The role of faecal calprotectin in the diagnosis of inflammatory bowel disease. BMJ. 2023 Feb 13;380:e068947. This lack of specificity limits its diagnostic role.
Fecal calprotectin is widely used in the primary care setting as a “rule out” test where IBD is unlikely in the presence of a normal calprotectin. In IBD it is used to monitor:[66]Ananthakrishnan AN, Adler J, Chachu KA, et al. AGA clinical practice guideline on the role of biomarkers for the management of crohn's disease. Gastroenterology. 2023 Dec;165(6):1367-99.
https://www.gastrojournal.org/article/S0016-5085(23)05064-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37981354?tool=bestpractice.com
[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[86]Mao R, Xiao YL, Gao x, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2012 Oct;18(10):1894-9.
http://www.ncbi.nlm.nih.gov/pubmed/22238138?tool=bestpractice.com
[87]Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015 Jun;110(6):802-19.
http://www.ncbi.nlm.nih.gov/pubmed/25964225?tool=bestpractice.com
[88]Rokkas T, Portincasa P, Koutroubakis IE. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta-analysis. J Gastrointestin Liver Dis. 2018 Sep;27(3):299-306.
https://www.jgld.ro/jgld/index.php/jgld/article/view/63
http://www.ncbi.nlm.nih.gov/pubmed/30240474?tool=bestpractice.com
relapse in patients with quiescent IBD, and
detection and monitoring of disease activity in symptomatic patients, potentially avoiding invasive and resource-intensive endoscopic monitoring.
Fecal calprotectin measurements may have a role in relapse prediction, assessment of treatment efficacy, and prediction of postsurgical relapse.[70]Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(suppl 3):s1-106.
https://gut.bmj.com/content/68/Suppl_3/s1.long
http://www.ncbi.nlm.nih.gov/pubmed/31562236?tool=bestpractice.com
[71]Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481-517.
https://journals.lww.com/ajg/fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29610508?tool=bestpractice.com
The test has also been proposed for use in postoperative CD.[89]Yamamoto T, Shimoyama T. Monitoring and detection of disease recurrence after resection for Crohn's disease: the role of non-invasive fecal biomarkers. Expert Rev Gastroenterol Hepatol. 2017 Oct;11(10):899-909.
http://www.ncbi.nlm.nih.gov/pubmed/28708427?tool=bestpractice.com
A fecal calprotectin value of <150 micrograms/g and normal C-reactive protein (CRP) suggests no active inflammation in patients in symptomatic remission; endoscopic assessment can be avoided in such patients. However, elevated biomarker levels in these patients warrant an endoscopic evaluation.[66]Ananthakrishnan AN, Adler J, Chachu KA, et al. AGA clinical practice guideline on the role of biomarkers for the management of crohn's disease. Gastroenterology. 2023 Dec;165(6):1367-99.
https://www.gastrojournal.org/article/S0016-5085(23)05064-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37981354?tool=bestpractice.com
In patients with CD with mild symptoms, biomarker levels alone cannot predict endoscopic activity, whereas in patients with moderate to severe symptoms, elevated fecal calprotectin or serum CRP suggests endoscopic activity and endoscopic evaluation can be avoided.[66]Ananthakrishnan AN, Adler J, Chachu KA, et al. AGA clinical practice guideline on the role of biomarkers for the management of crohn's disease. Gastroenterology. 2023 Dec;165(6):1367-99.
https://www.gastrojournal.org/article/S0016-5085(23)05064-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37981354?tool=bestpractice.com

 [Figure caption and citation for the preceding image starts]: CT scan demonstrating thickening of the terminal ileum in a patient with Crohn disease exacerbationProvided by Drs Wissam Bleibel, Bishal Mainali, Chandrashekhar Thukral, and Mark A. Peppercorn, the previous authors of this topic [Citation ends].
[Figure caption and citation for the preceding image starts]: CT scan demonstrating thickening of the terminal ileum in a patient with Crohn disease exacerbationProvided by Drs Wissam Bleibel, Bishal Mainali, Chandrashekhar Thukral, and Mark A. Peppercorn, the previous authors of this topic [Citation ends].
