Etiology
The etiopathogenesis of Crohn disease (CD) is multifactorial and includes genetic and environmental factors.
Genetic factors
Genome-wide association studies have identified over 71 different genetic susceptibility loci, with the strongest associations being between CARD15 (caspase recruitment domain family, member 15), which encodes the NOD2 (nucleotide-binding oligomerization domain containing 2) pathogen recognition protein, and other loci, such as the IBD5 locus, the autophagy gene ATG16L1 (ATG16 autophagy 16-like 1), and the interleukin-23 receptor.[27][28][29] Genotype may also influence the distribution of CD.[30]
Susceptibility loci and identified genetic risk factors account for <20% of heritable risk for inflammatory bowel disease.[31][32][33]
Environmental factors
Include smoking, birth control pill, nonsteroidal anti-inflammatory drugs, exposure to antibiotics, diet (low fruit, low vegetable, low fiber, high refined sugar, high ultra-processed foods, high meat, low dairy), sedentary lifestyle, and not being breastfed.[22][23][24][25][26][34][35][36][37][38][39][40][41][42][43]
Campylobacter species have been associated with incident and active inflammatory bowel disease, including CD.[44][45]
Some studies suggest that Mycobacterium avium paratuberculosis may contribute to increased risk for CD.[46] However, a coincidental association has not been excluded.[47][48]
Vitamin D deficiency and zinc deficiency may complicate CD; a causative role has not been established.[37]
Pathophysiology
Current theories regarding the pathophysiology of CD indicate a role for infectious, immunologic, environmental, dietary, and psychosocial factors in a genetically and immunologically susceptible individual.[1][21][22][36][37][49][50]
The initial lesion starts as an inflammatory infiltrate around intestinal crypts that subsequently develops into ulceration of the superficial mucosa. The inflammation progresses to involve deeper layers and can form noncaseating granulomas. These granulomas involve all layers of the intestinal wall and the mesentery and regional lymph nodes. The finding of these granulomas is highly suggestive of CD, yet their absence does not exclude the diagnosis.[51][52]
Early endoscopic findings include hyperemia and edema of the inflamed mucosa. This progresses to discrete deep ulcers located transversely and longitudinally, creating a cobblestone appearance. These lesions are sometimes separated by normal bowel mucosa in a discontinuous fashion (skip lesions).[4][Figure caption and citation for the preceding image starts]: Endoscopic view of Crohn ileitisProvided by Drs Wissam Bleibel, Bishal Mainali, Chandrashekhar Thukral, and Mark A. Peppercorn, the previous authors of this topic [Citation ends].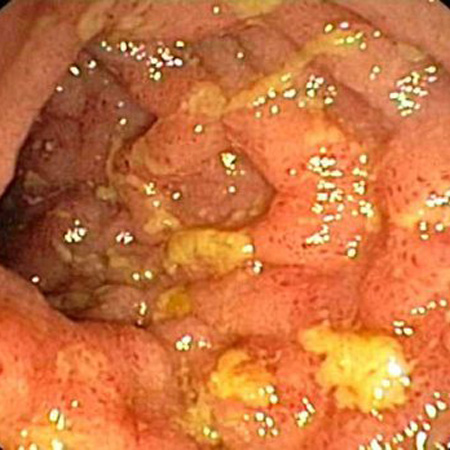 [Figure caption and citation for the preceding image starts]: Endoscopic view of normal terminal ileumFrom the personal collection of Dr Charlotte Ford, North Middlesex Hospital Trust, London, UK [Citation ends].
[Figure caption and citation for the preceding image starts]: Endoscopic view of normal terminal ileumFrom the personal collection of Dr Charlotte Ford, North Middlesex Hospital Trust, London, UK [Citation ends].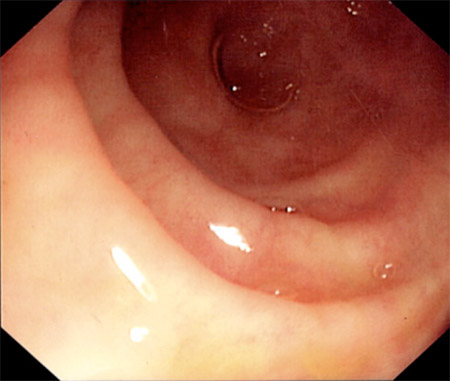
CD is a relapsing remitting disease with a pattern of cumulative bowel damage. Continuous active inflammation increases the probability of complications. Chronic transmural inflammation thickens the bowel wall and leads to scarring, luminal narrowing, and stricture formation. This may lead to fistulization, sinus tracts formation, perforation, and/or abscess formation. Chronic inflammation also damages the intestinal mucosa, resulting in deficient absorptive ability. This can lead to malnutrition, dehydration, and vitamin and nutrient deficiencies.[10]
Involvement of the terminal ileum interferes with bile acid absorption, which can lead to diarrhea, fat-soluble vitamin deficiency, and gallstone formation.[7][53][54] Excessive fat in the stool binds to calcium, thereby increasing oxalate absorption and predisposing to oxalate kidney stone formation.[55]
In addition to manifestations related to the gastrointestinal tract, CD may involve multiple extraintestinal organs and systems including skin, joints, mouth, eyes, liver, and bile ducts.[4][5][6][7] Some of these disorders have autoimmune mechanisms.[4][5]
Classification
Vienna classification of CD[2]
Classifies patients with CD into 24 subgroups.
Age at diagnosis - when first definitively established by radiology, endoscopy, pathology, or surgery.
A1 <40 years of age.
A2 40 years of age or more
Location - maximum extent of disease involvement at any time before the first resection. Minimum involvement for a location is aphthous lesion or ulceration. Both small- and large-bowel exams are required for classification.
L1 - terminal ileum - limited to terminal ileum.
L2 - colon - any colonic location between the cecum and rectum, with no small bowel or upper gastrointestinal (GI) involvement.
L3 - ileocolonic - disease of ileum and any location between the ascending colon and rectum.
L4 - upper gastrointestinal - any disease proximal to the terminal ileum, regardless of additional involvement of the terminal ileum or colon.
Behavior
B1 - nonstricturing, nonpenetrating.
B2 - stricturing - constant luminal narrowing demonstrated by radiologic, endoscopic, or surgical-pathologic methods, with prestenotic dilation or obstructive signs/symptoms, without the presence of penetrating disease, at any time in the course of the disease.
B3 - penetrating - occurrence of intra-abdominal or perianal fistulae, inflammatory masses, and/or abscesses at any time in the course of the disease. Perianal ulcers are included. Postoperative intra-abdominal complications and skin tags are excluded.
Montreal classification of CD[3]
The Montreal revision of the Vienna classification does not change the three predominant parameters, but introduces modifications to each category to allow for: early onset; upper GI disease to coexist with more distal disease; the separation of perianal disease into a subclassification; a stipulated time to be set before disease behavior is classified.
Age at diagnosis
A1 below 16 years
A2 between 17 and 40 years
A3 above 40 years
Location
L1 ileal
L2 colonic
L3 ileocolonic
L4 isolated upper disease (L4 is a modifier that can be added to L1 to L3 when concomitant upper gastrointestinal disease is present)
Behavior
B1 nonstricturing, nonpenetrating
B2 stricturing
B3 penetrating
P peripheral disease modifier (added to B1 to B3 when concomitant perianal disease is present)
Use of this content is subject to our disclaimer