Patients often present with a history of self-harm by ingestion. Most patients will state in the history that they have ingested acetaminophen or an acetaminophen-containing combination product. Some patients will not be certain which tablets or pills they have taken, and witness corroboration may be important. Others may be unaware that the preparations ingested in overdose contained acetaminophen. Brand name products are periodically reformulated; for example, some manufacturers that have traditionally made nonsteroidal anti-inflammatory (NSAID)-containing products have begun to manufacture products that contain acetaminophen. When possible, clinicians should make every attempt to verify the exact ingredients in the products the patient is taking. Patients with painful conditions may have taken repeated over-the-counter analgesics for pain relief, resulting in supratherapeutic ingestion of acetaminophen.[15]Daly FF, O'Malley GF, Heard K, et al. Prospective evaluation of repeated supratherapeutic acetaminophen (paracetamol) ingestion. Ann Emerg Med. 2004 Oct;44(4):393-8.
http://www.ncbi.nlm.nih.gov/pubmed/15459622?tool=bestpractice.com
[16]Heard K, Sloss D, Weber S, et al. Overuse of over-the-counter analgesics by emergency department patients. Ann Emerg Med. 2006 Sep;48(3):315-8.
http://www.ncbi.nlm.nih.gov/pubmed/16934651?tool=bestpractice.com
[18]Kaufman DW, Kelly JP, Battista DR, et al. Five-year trends in acetaminophen use exceeding the recommended daily maximum dose. Br J Clin Pharmacol. 2019 May;85(5):1028-34.
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.13894
http://www.ncbi.nlm.nih.gov/pubmed/30740763?tool=bestpractice.com
[36]Hawkins LC, Edwards JN, Dargan PI. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature. Drug Saf. 2007;30:465-479.
http://www.ncbi.nlm.nih.gov/pubmed/17536874?tool=bestpractice.com
It is essential to attempt to establish the timing of the overdose as this will affect subsequent patient management.
Acetaminophen overdose should be suspected in anyone who has taken an overdose of any substances or who has self-harmed in any way. It is equally important to seek evidence of other substance ingestion in patients who have presented with acetaminophen overdose. All patients suspected of having an overdose should have a serum level of acetaminophen checked.
Overall clinical picture
Patients who present within the first 24 hours after ingestion of a potentially toxic dose of acetaminophen may be asymptomatic. Early nonspecific signs that may be present in the first 24 hours include anorexia, malaise, nausea, vomiting, or vague abdominal pain.[5]Fontana RJ, Liou I, Reuben A, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2023 Mar 1;77(3):1036-65.
https://journals.lww.com/hep/fulltext/2023/03000/aasld_practice_guidance_on_drug,_herbal,_and.28.aspx
Patients may present with reduced consciousness level or coma if they have ingested a combined preparation of acetaminophen and an opioid, have co-ingested alcohol or other drugs that cause depressed mental status, or who present after a high-risk ingestion (>30 g or an acetaminophen concentration above the high-risk line on the nomogram).[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062
http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
In a small percentage of patients, toxicity progresses and clinical signs of hepatic injury become apparent. Patients may manifest right upper quadrant pain and tenderness, hepatomegaly, nausea, vomiting, and jaundice. Serum aspartate aminotransferase (AST) elevation usually begins around 24 hours to 36 hours post ingestion.[51]Green TJ, Sivilotti MLA, Langmann C, et al. When do the aminotransferases rise after acute acetaminophen overdose? Clin Toxicol (Phila). 2010;48:787-792.
http://www.ncbi.nlm.nih.gov/pubmed/20969501?tool=bestpractice.com
[52]Singer AJ, Carracio TR, Mofenson HC. The temporal profile of increased transaminase levels in patients with acetaminophen-induced liver dysfunction. Ann Emerg Med. 1995 Jul;26(1):49-53.
http://www.ncbi.nlm.nih.gov/pubmed/7793720?tool=bestpractice.com
Liver injury usually becomes maximal at 72-96 hours and, dependent on the severity of the ingestion, may be characterized by massive elevation of serum AST and alanine aminotransferase (ALT) activity, hyperbilirubinemia, and prolongation of the prothrombin time. Nephrotoxicity occasionally occurs during this timeframe. Although most patients will recover, some may progress to have fulminant hepatic failure with encephalopathy, coma, renal failure (hepatorenal syndrome), and rarely, hemorrhage.[53]Prescott LF, Critchley JA. The treatment of acetaminophen poisoning. Annu Rev Pharmacol Toxicol. 1983;23:87-101.
http://www.ncbi.nlm.nih.gov/pubmed/6347057?tool=bestpractice.com
[54]Flanagan RJ, Mant TG. Coma and metabolic acidosis early in severe acute paracetamol poisoning. Hum Toxicol. 1986 May;5(3):179-82.
http://www.ncbi.nlm.nih.gov/pubmed/3710495?tool=bestpractice.com
[55]Roth B, Woo O, Blanc P. Early metabolic acidosis and coma after acetaminophen ingestion. Ann Emerg Med. 1999 Apr;33(4):452-6.
http://www.ncbi.nlm.nih.gov/pubmed/10092726?tool=bestpractice.com
[56]Hamlyn AN, Douglas AP, James O. The spectrum of paracetamol (acetaminophen) overdose: clinical and epidemiological studies. Postgrad Med J. 1978 Jun;54(632):400-4.
https://pmj.bmj.com/content/postgradmedj/54/632/400.full.pdf
http://www.ncbi.nlm.nih.gov/pubmed/683908?tool=bestpractice.com
[57]Prescott LF, Proudfoot AT, Cregeen RJ. Paracetamol-induced acute renal failure in the absence of fulminant liver damage. Br Med J (Clin Res Ed). 1982 Feb 6;284(6313):421-2.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1495982/pdf/bmjcred00592-0059e.pdf
http://www.ncbi.nlm.nih.gov/pubmed/6800485?tool=bestpractice.com
Abnormalities of prothrombin time, glucose, lactate, creatinine, pH, and phosphate, are more important markers of prognosis than the degree aminotransferase elevation.[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45.
http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com
[59]Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003 Jan;31(1):299-305.
http://www.ncbi.nlm.nih.gov/pubmed/12545033?tool=bestpractice.com
Liver biopsies and post-mortem studies reveal extensive centrilobular hepatic necrosis without inflammatory changes.[60]Davidson DG, Eastham WN. Acute liver necrosis following overdose of paracetamol. Br Med J. 1966 Aug 27;2(5512):497-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1943529/pdf/brmedj02356-0031.pdf
http://www.ncbi.nlm.nih.gov/pubmed/5913083?tool=bestpractice.com
[61]Clark R, Borirakchanyavat V, Davidson AR, et al. Hepatic damage and death from overdose of paracetamol. Lancet. 1973 Jan 13;1(7794):66-70.
http://www.ncbi.nlm.nih.gov/pubmed/4118649?tool=bestpractice.com
[62]Lesna M, Watson AJ, Douglas AP, et al. Evaluation of paracetamol-induced damage in liver biopsies: acute changes and follow-up findings. Virchows Arch A Pathol Anat Histol. 1976 Jul 21;370(4):333-44.
http://www.ncbi.nlm.nih.gov/pubmed/826016?tool=bestpractice.com
[63]Portmann B, Talbot IC, Day DW, et al. Histopathological changes in the liver following a paracetamol overdose: correlation with clinical and biochemical parameters. J Pathol. 1975 Nov;117(3):169-81.
http://www.ncbi.nlm.nih.gov/pubmed/1214189?tool=bestpractice.com
Renal injury is rare, and when it occurs, serum creatinine typically begins to rise soon after serum aminotransferase activity has peaked. It is rare for patients to present with isolated renal injury (<1% of cases); however, renal injury is common in patients with significant hepatotoxicity and hepatic failure, occurring in 25% and 53% of cases, respectively.[6]Prescott LF. Paracetamol overdosage. Pharmacological considerations and clinical management. Drugs. 1983 Mar;25(3):290-314.
http://www.ncbi.nlm.nih.gov/pubmed/6343056?tool=bestpractice.com
[7]Stollings JL, Wheeler AP, Rice TW. Incidence and characterization of acute kidney injury after acetaminophen overdose. J Crit Care. 2016 Oct;35:191-4.
http://www.ncbi.nlm.nih.gov/pubmed/27481758?tool=bestpractice.com
[8]Wilkinson SP, Moodie H, Arroyo VA, et al. Frequency of renal impairment in paracetamol overdose compared with other causes of acute liver damage. J Clin Pathol. 1977 Feb;30(2):141-3.
http://www.ncbi.nlm.nih.gov/pubmed/845262?tool=bestpractice.com
Early presentation with coma and severe metabolic acidosis without hepatotoxicity is also rare and is associated with massive overdose (serum acetaminophen level >800 micrograms/mL).[54]Flanagan RJ, Mant TG. Coma and metabolic acidosis early in severe acute paracetamol poisoning. Hum Toxicol. 1986 May;5(3):179-82.
http://www.ncbi.nlm.nih.gov/pubmed/3710495?tool=bestpractice.com
[55]Roth B, Woo O, Blanc P. Early metabolic acidosis and coma after acetaminophen ingestion. Ann Emerg Med. 1999 Apr;33(4):452-6.
http://www.ncbi.nlm.nih.gov/pubmed/10092726?tool=bestpractice.com
Laboratory tests
Depending on the time of presentation relative to the time of acetaminophen overdose, laboratory investigation may show a measurable serum acetaminophen concentration, abnormal serum AST or ALT, abnormal prothrombin time or international normalized ratio (INR), renal dysfunction, or metabolic acidosis. Initial laboratory investigations should include:[53]Prescott LF, Critchley JA. The treatment of acetaminophen poisoning. Annu Rev Pharmacol Toxicol. 1983;23:87-101.
http://www.ncbi.nlm.nih.gov/pubmed/6347057?tool=bestpractice.com
[54]Flanagan RJ, Mant TG. Coma and metabolic acidosis early in severe acute paracetamol poisoning. Hum Toxicol. 1986 May;5(3):179-82.
http://www.ncbi.nlm.nih.gov/pubmed/3710495?tool=bestpractice.com
[55]Roth B, Woo O, Blanc P. Early metabolic acidosis and coma after acetaminophen ingestion. Ann Emerg Med. 1999 Apr;33(4):452-6.
http://www.ncbi.nlm.nih.gov/pubmed/10092726?tool=bestpractice.com
[56]Hamlyn AN, Douglas AP, James O. The spectrum of paracetamol (acetaminophen) overdose: clinical and epidemiological studies. Postgrad Med J. 1978 Jun;54(632):400-4.
https://pmj.bmj.com/content/postgradmedj/54/632/400.full.pdf
http://www.ncbi.nlm.nih.gov/pubmed/683908?tool=bestpractice.com
[57]Prescott LF, Proudfoot AT, Cregeen RJ. Paracetamol-induced acute renal failure in the absence of fulminant liver damage. Br Med J (Clin Res Ed). 1982 Feb 6;284(6313):421-2.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1495982/pdf/bmjcred00592-0059e.pdf
http://www.ncbi.nlm.nih.gov/pubmed/6800485?tool=bestpractice.com
Serum acetaminophen levels
For acute acetaminophen overdose, specific management is dependent on the serum acetaminophen level relative to the time of ingestion.
A timed serum acetaminophen level is drawn at least 4 hours after ingestion to risk-stratify likelihood of liver injury and the need for acetylcysteine treatment.[2]Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975 Jun;55(6):871-6.
http://www.ncbi.nlm.nih.gov/pubmed/1134886?tool=bestpractice.com
The serum level is plotted on the Rumack-Matthew nomogram to determine whether treatment with acetylcysteine is required.
This nomogram was developed by plotting timed acetaminophen concentrations from acetaminophen overdose patients on a graph, and drawing a line to discriminate those who did and those who did not develop hepatotoxicity (defined as aminotransferase levels >1000 IU/L).[2]Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975 Jun;55(6):871-6.
http://www.ncbi.nlm.nih.gov/pubmed/1134886?tool=bestpractice.com
The nomogram was later updated to contain a line that connects a point at 150 micrograms/mL at 4 hours after ingestion and 4.7 micrograms/mL at 24 hours after ingestion, termed the "treatment line.” This line is 25% lower than the original nomogram line and was added at the Food and Drug Administration’s request for additional safety during the first US open-label study.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062
http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
[3]Rumack BH, Peterson RC, Koch GG, et al. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981 Feb 23;141(3 spec no):380-5.
http://www.ncbi.nlm.nih.gov/pubmed/7469629?tool=bestpractice.com
[64]Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40(1):3-20.
http://www.ncbi.nlm.nih.gov/pubmed/11990202?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Revised Rumack-Matthew Nomogram for the Acute Ingestion of AcetaminophenDart RC et al. JAMA Netw Open. 2023;6(8):e2327739; used with permission [Citation ends].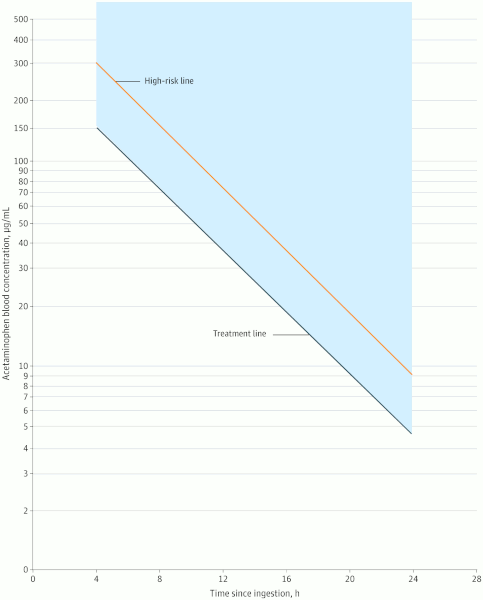
In 2023, the revised Rumack-Matthew nomogram was developed, containing only lines that guide clinical action.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062
http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
As well as the “treatment line,” the revised Rumack-Matthew nomogram also contains a “high risk” line that connects a point at 300 micrograms/mL at 4 hours and 9.4 micrograms/mL at 24 hours after ingestion.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062
http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
[4]Smilkstein MJ, Knapp GL, Kulig KW, et al. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988 Dec 15;319(24):1557-62.
http://www.ncbi.nlm.nih.gov/pubmed/3059186?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Revised Rumack-Matthew Nomogram for the Acute Ingestion of AcetaminophenDart RC et al. JAMA Netw Open. 2023;6(8):e2327739; used with permission [Citation ends].
Acetylcysteine treatment is started if the acetaminophen level falls on or above the treatment line.
The nomogram is not applicable in the following circumstances: unknown time of ingestion, repeated supratherapeutic ingestions, late presenting patients (patients presenting greater than 24 hours after ingestion), and those with evidence of hepatotoxicity despite undetectable or therapeutic acetaminophen level. Some have suggested that co-ingestants that may delay gastrointestinal absorption (e.g., anticholinergic drugs) may also limit the nomogram, but there are no systematic studies to support this. Some experts recommend obtaining a second acetaminophen concentration 4-6 hours after the first, if the first measurement is greater than 10 micrograms/mL.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062
http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
AST and ALT levels
Levels are done initially and repeated in the course of patient management (usually every 12-24 hours). The degree of derangement in laboratory results depends on the time of presentation relative to the time of acetaminophen overdose.
The acetaminophen-aminotransferase multiplication product, calculated by multiplying the serum acetaminophen concentration by the aminotransferase activity (AST or ALT, whichever is higher), can potentially complement early assessment of patients who are at low risk of hepatotoxicity.[65]Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol (Phila). 2017 Sep;55(8):879-92.
http://www.ncbi.nlm.nih.gov/pubmed/28447858?tool=bestpractice.com
Patients with a multiplication product greater than 10,000 mg/L × IU/L have a high likelihood of developing hepatotoxicity, especially if it is more than 8 hours post ingestion; patients with a multiplication product lower than 1500 mg/L × IU/L have a low likelihood of developing hepatotoxicity.[66]Wong A, Sivilotti ML, Dargan PI, et al. External validation of the paracetamol-aminotransferase multiplication product to predict hepatotoxicity from paracetamol overdose. Clin Toxicol (Phila). 2015;53(8):807-14.
http://www.ncbi.nlm.nih.gov/pubmed/26175095?tool=bestpractice.com
[67]Wong A, Sivilotti MLA, Graudins A. Accuracy of the paracetamol-aminotransferase multiplication product to predict hepatotoxicity in modified-release paracetamol overdose. Clin Toxicol (Phila). 2017 Jun;55(5):346-51.
http://www.ncbi.nlm.nih.gov/pubmed/28421844?tool=bestpractice.com
[68]Wong A, Sivilotti MLA, Gunja N, et al. Accuracy of the paracetamol-aminotransferase product to predict hepatotoxicity in paracetamol overdose treated with a 2-bag acetylcysteine regimen. Clin Toxicol (Phila). 2018 Mar;56(3):182-8.
http://www.ncbi.nlm.nih.gov/pubmed/28756679?tool=bestpractice.com
Prothrombin time/INR, arterial pH, arterial lactate level, and metabolic panel with serum creatinine
These are used to monitor severity of hepatic failure and assist in patient stratification for optimal benefit in orthotopic liver transplantation (e.g., using the King’s College Criteria).[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45.
http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com
[69]O'Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014 Mar;60(3):663-70.
https://www.journal-of-hepatology.eu/article/S0168-8278(13)00750-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/24211740?tool=bestpractice.com
May not be indicated in early presenting, low-risk patients; AST abnormalities always precede evidence of hepatic synthetic dysfunction.
A prolonged INR is a poor prognostic sign.[5]Fontana RJ, Liou I, Reuben A, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2023 Mar 1;77(3):1036-65.
https://journals.lww.com/hep/fulltext/2023/03000/aasld_practice_guidance_on_drug,_herbal,_and.28.aspx
Renal injury may rarely occur in the absence of significant hepatotoxicity, and is relatively common with significant hepatotoxicity.
Other tests to consider
Serum salicylate level should be considered in patients who have ingested substances in an attempt at self-harm.
Ethanol level and blood glucose level should be obtained in any patient with altered mental status.
Elevated lipase consistent with pancreatitis may occur, especially in patients with alcohol-use disorder.
Phosphate may be helpful for prognostication for patients with acute liver failure as a supplement to the King's College Criteria. Elevated phosphate is associated with worse outcomes.[70]Baquerizo A, Anselmo D, Shackleton C, et al. Phosphorus ans an early predictive factor in patients with acute liver failure. Transplantation. 2003 Jun 27;75(12):2007-14.
https://journals.lww.com/transplantjournal/fulltext/2003/06270/phosphorus_ans_an_early_predictive_factor_in.15.aspx
http://www.ncbi.nlm.nih.gov/pubmed/12829902?tool=bestpractice.com