Criteria
Toxic dose
A supratherapeutic dose is considered to be >4 g within a 24-hour period.
Acute overdose
An acute acetaminophen overdose in adults, in terms of Food and Drug Administration (FDA)-labeled therapeutic dosing, is minimally defined as a cumulative dose of acetaminophen >4 g and is ingested over 8 hours or less (some authors use a period of 4 hours). In adults and adolescents, hepatotoxicity may occur following ingestion of ≥10 g in 24 hours. Children are at risk for hepatotoxicity with acute acetaminophen ingestions ≥150 mg/kg in a single 24 hour period.[1]
Serum acetaminophen level
For acute acetaminophen overdose, specific management is dependent on the serum acetaminophen level relative to the time of ingestion. A timed serum acetaminophen level is drawn at least 4 hours after ingestion to risk-stratify likelihood of liver injury and the need for acetylcysteine treatment.[2]
The serum level is plotted on the Rumack-Matthew nomogram to determine whether treatment with antidote (acetylcysteine) is required.
This nomogram was developed by plotting timed acetaminophen concentrations from acetaminophen overdose patients on a figure, and drawing a line to discriminate those who did and those who did not develop hepatotoxicity (defined as aminotransferase levels >1000 IU/L).
The line connects a point at 150 micrograms/mL at 4 hours after ingestion and 4.7 micrograms/mL at 24 hours after ingestion and is termed the "treatment line." It is 25% more conservative than the nomogram as originally developed. The revised Rumack-Matthew nomogram also contains a “high risk” line that connects a point at 300 micrograms/mL at 4 hours and 9.4 micrograms/mL at 24 hours after ingestion.[1]
Acetylcysteine treatment is started if the acetaminophen level falls on or above the line.[Figure caption and citation for the preceding image starts]: Revised Rumack-Matthew Nomogram for the Acute Ingestion of AcetaminophenDart RC et al. JAMA Netw Open. 2023;6(8):e2327739; used with permission [Citation ends].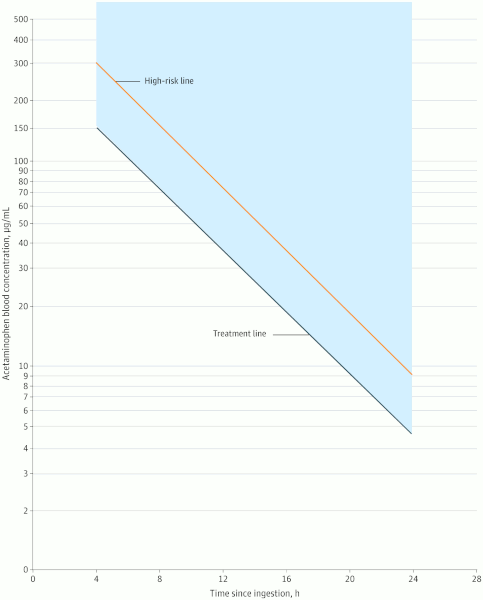
The nomogram is not applicable in the following circumstances:
Unknown time of ingestion
Repeated supratherapeutic ingestions
Late presenting patients
Those with evidence of hepatotoxicity despite undetectable or therapeutic acetaminophen levels.
Co-ingestion of anticholinergic products may delay gastric absorption. However, the effects of this on the diagnostic accuracy of the nomogram are not known. Providers should consider the possibility of delayed absorption in patients who have anticholinergic symptoms.
Repeated supratherapeutic ingestion
Repeated supratherapeutic acetaminophen ingestion denotes ingestion of excess acetaminophen with intent to treat pain or fever (i.e., without self-harm intent). It may be accidental or intentional.
Repeated supratherapeutic ingestion is defined as multiple episodes of acetaminophen ingestion over a period greater than 24 hours that results in any of the following: cumulative dose of >4 g/day.[1][44]
Findings consistent with acetaminophen toxicity (repeated vomiting, right upper quadrant abdominal tenderness, or mental status changes)
Ingestion period of 24-48 hours: >6 g/day or >150 mg/kg/day (whichever is less)
Ingestion period of greater than 48 hours: >4 g/day or >100 mg/kg/day (whichever dose is less)
Acetaminophen-aminotransferase multiplication product
Risk stratification tools commonly used in acetaminophen overdose, such as King’s College Criteria, identify patients who are at high risk after hepatotoxicity becomes clinically apparent. Additionally, these criteria do not identify patients who are at low risk of hepatotoxicity. The acetaminophen-aminotransferase multiplication product, calculated by multiplying the serum acetaminophen concentration by the aminotransferase activity (aspartate aminotransferase [AST] or alanine aminotransferase [ALT], whichever is higher), can potentially complement early assessment of these patients.[65]
Patients with a multiplication product greater than 10,000 mg/L × IU/L have a high likelihood of developing hepatotoxicity, especially if it is more than 8 hours post ingestion; patients with a multiplication product lower than 1500 mg/L × IU/L have a low likelihood of developing hepatotoxicity.[66][67][68]
Hepatotoxicity
The definition of hepatotoxicity after acetaminophen overdose is a serum AST or ALT of 1000 IU/L or greater.
Hepatic encephalopathy: West Haven classification system[72]
Grade 1: trivial lack of awareness; euphoria or anxiety; shortened attention span; plus impaired performance.
Grade 2: lethargy or apathy; minimal disorientation to time and place; inappropriate behavior; subtle personality changes; impaired performance in subtraction.
Grade 3: somnolence but with responsiveness to verbal stimuli; marked confusion; gross disorientation about time and place.
Grade 4: coma.
Use of this content is subject to our disclaimer