Approach
A diagnosis of preeclampsia should be made if there is persistent, new-onset hypertension, usually with proteinuria, after 20 weeks' gestation.[1][2][16][53] The absence of hypertension excludes the diagnosis, although there are related conditions, such as HELLP syndrome, that may present with and without hypertension. HELLP syndrome is a subtype of severe preeclampsia characterized by hemolysis (H), elevated liver enzymes (EL), and low platelets (LP).[16] The presence of proteinuria is no longer mandatory in the diagnosis of preeclampsia, however.[2] A diagnosis of preeclampsia can be made in the absence of proteinuria if hypertension presents with either maternal systemic involvement, usually hepatic or hematological complications, or fetal growth restriction.[1][2] After diagnosis, fetal assessment should be performed with further maternal tests to assess systemic involvement.
If there are signs and symptoms of severe preeclampsia or complications, immediate treatment is needed. On confirmation of the diagnosis, or if there are concerns regarding maternal or fetal health, the woman should be admitted to an obstetric care facility for management.[1][16]
History
Preeclampsia usually occurs in pregnant women after 20 weeks' gestation.[1][16][54] Key risk factors include nulliparity, previous maternal history or family history, body mass index >30, maternal age >40 years, multiple (twin) pregnancy, subfertility, gestational hypertension (hypertension developing after 20 weeks' gestation in the absence of both proteinuria and systemic symptoms), preexisting diabetes, polycystic ovary syndrome, autoimmune disease, renal disease, preexisting cardiovascular disease and chronic hypertension.
The woman may be asymptomatic and diagnosed at a routine clinic visit, or she may present acutely with the following symptoms.
Headache: usually frontal; presence of this symptom classifies preeclampsia as severe.[1] Occurs in around 40% of women with severe disease, and is one of the few symptoms that predict an increased risk of eclampsia.[8]
Upper abdominal pain: usually right upper quadrant pain; occurs in around 16% of women with severe disease, and is a clinical symptom of HELLP syndrome.[8]
Visual disturbances: photopsia (perceived flashing lights in the visual fields), scotomata, and retinal vasospasm are relatively rare, but predict an increased risk of eclampsia. Cortical blindness should alert the clinician to underlying cerebral edema.
Breathlessness: due to pulmonary edema and may complicate preeclampsia. If it occurs after delivery, it is one of the main causes of maternal mortality.
Seizures: mandates admission to intensive care unit, stabilization, and delivery.[1]
Oliguria: may be associated with increasing edema. Women are at most risk postpartum, when pulmonary edema is more likely.
The presence of these symptoms, in addition to hypertension with or without proteinuria, classifies preeclampsia as severe.[1] If fetal movements are reduced, there is a need for immediate fetal wellbeing assessment.
[  ]
]
Physical exam
Hypertension (defined as systolic blood pressure [BP] ≥140 mmHg and/or diastolic BP ≥90 mmHg) in a previously normotensive woman is diagnostic.[1][16][54] At least two measurements should be made, at least 4 hours apart.[1]
Edema is very common, but is not discriminatory, and so should not be used in diagnosis. Hyper-reflexia and/or clonus are rare and have little value in the clinical assessment. Fundoscopy is rarely abnormal, but, if it is, underlying chronic hypertension is implied.
If the uterus is small for dates, this implies that the amniotic fluid volume is reduced, which may signify growth restriction, and fetal ultrasound assessment is required.
[  ]
Fetal growth restriction is found in around 30% of women with preeclampsia.[8]
]
Fetal growth restriction is found in around 30% of women with preeclampsia.[8]
Urinalysis
Reagent strip testing can be used to screen for the presence of protein in the urine. Reagent strip testing with automated readers is more accurate than visual analysis.
Proteinuria in association with elevated blood pressure in the preeclampsia range requires referral to a specialist unit or hospital admission for assessment. In the absence of proteinuria or systemic signs of preeclampsia, an alternative diagnosis should be sought.
Urinary protein can be estimated in a 24-hour urine collection, with a diagnostic level considered to be urinary excretion of ≥300 mg protein/24 hours.[1][2][31]
However, completing a 24-hour urine collection is awkward for women, and the UK National Institute for Health and Care Excellence (NICE) recommends not routinely using 24-hour urine collection to quantify proteinuria in pregnant women.[16] If available, an alternative spot test such as the protein:creatinine ratio (PCR, for which a result of ≥0.3 is diagnostic) is preferred.[1][16][31][54] If the proteinuria measurement is above the diagnostic threshold but there is still diagnostic uncertainty, consider retesting on a new sample in addition to clinical review.
Because the level of proteinuria does not correlate with outcome, there is no benefit in routinely repeating urinalysis once a diagnosis has been made.[16]
Fetal assessment
Immediate fetal ultrasound assessment is required if fetal movements are reduced or fetal growth restriction is suspected.
[  ]
Fetal biometry should be used to diagnose or exclude fetal growth restriction, although growth can only be fully assessed by scans performed 2 weeks apart.
]
Fetal biometry should be used to diagnose or exclude fetal growth restriction, although growth can only be fully assessed by scans performed 2 weeks apart.
Other methods of fetal assessment should be used in all women initially using the following tests:[1][16][54]
Umbilical artery Doppler velocimetry is the main assessment tool. It reduces perinatal mortality and supports better decision-making, leading to more appropriate delivery decisions. It should be carried out on admission and, if normal, repeated twice weekly. If abnormal, more intensive monitoring may be required using other means, including Doppler assessment of other fetal vessels and fetal cardiotocography. [
 ]
Delivery may be necessary within a few days.[Figure caption and citation for the preceding image starts]: Umbilical artery Doppler velocimetry: (1) normal pattern; (2) reduced end diastolic flow; (3) absent end diastolic flow; (4) reverse end diastolic flowFrom the personal collection of Dr James J. Walker; used with permission [Citation ends].
]
Delivery may be necessary within a few days.[Figure caption and citation for the preceding image starts]: Umbilical artery Doppler velocimetry: (1) normal pattern; (2) reduced end diastolic flow; (3) absent end diastolic flow; (4) reverse end diastolic flowFrom the personal collection of Dr James J. Walker; used with permission [Citation ends].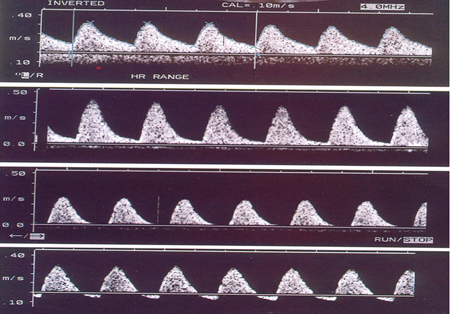
Fetal cardiotocography is recommended to assess fetal wellbeing, but is of little prognostic value. It should be performed initially, and then no more than twice weekly, unless there is a cause for concern such as vaginal bleeding, reduced fetal movements, or increased severity of disease. [
 ]
]
Amniotic fluid assessment, the single deepest vertical pocket being preferred over the amniotic index. Can easily be combined with umbilical artery Doppler velocimetry.
Other maternal investigations
Complete blood count, serum creatinine, and liver function tests (LFTs) are all useful indicators of disease progression, and are therefore recommended in all women after initial urinalysis and fetal assessment.
Elevated serum creatinine implies underlying renal disease. Although elevated serum uric acid is associated with severe preeclampsia, it does not offer additional diagnostic value. Decreased platelet and increased transaminase levels are partly diagnostic for HELLP syndrome. The platelet count is the main criterion used in classifying severity of HELLP syndrome. If the platelet count is <100 × 10⁹/L, a full coagulation screen and LFTs should be carried out. If the platelet count is ≥100 × 10⁹/L, further coagulation tests are not typically recommended.
Placental growth factor (PlGF) testing helps to assess the risk of preeclampsia and plan for early and safe delivery, if indicated. The test also helps to identify women who are not at risk of preeclampsia, thereby reducing unnecessary monitoring and hospital attendances. Testing, therefore, enables clinical teams to target resources to keep these women under appropriate follow-up. NICE advocates the use of PlGF testing on one occasion to help decide on care (to help rule in or rule out preeclampsia) if there is a suspicion of preeclampsia in women presenting between 20 weeks to 34 weeks plus 6 days of gestation.[16][55][56] A low PlGF test result (<100 pg/mL) does not always mean a woman has preeclampsia, because the result can be associated with other conditions affecting the placenta. However, PlGF-based test results are useful for decision-making alongside clinical judgment, particularly for women who had hypertension or proteinuria before becoming pregnant and those at higher risk of severe adverse pregnancy outcomes (e.g., women from African, Caribbean, or Asian family backgrounds). One cluster-randomized controlled trial (the PARROT study) reported faster time to diagnosis and lower incidence of severe maternal adverse outcomes in maternity units in which PlGF results were available, compared with those units in which PlGF results were concealed (control).[57] Median time to preeclampsia diagnosis was 4.1 days with concealed testing versus 1.9 days with revealed testing (time ratio 0.36, 95% confidence interval [CI] 0.15 to 0.87; P=0.027). Maternal severe adverse outcomes were reported in 24 (5%) of 447 women in the concealed testing group versus 22 (4%) of 573 women in the revealed testing group (adjusted odds ratio 0.32, 95% CI 0.11 to 0.96; P=0.043). Perinatal outcomes were not affected.[57] NICE also recommends the maternal serum soluble fms-like tyrosine kinase 1 (sFlt-1) to PlGF ratio as an alternative to PlGF-only testing, although the need for traditional history taking and exam remains paramount.[55] The INSPIRE prospective, interventional, parallel-group, randomized clinical trial found the use of the sFlt-1/PlGF ratio in women presenting with suspected preeclampsia significantly improved clinical precision without affecting the hospital admission rate.[58] The number of admissions was not significantly different between groups. However, the reveal (sFlt-1/PlGF result known to clinicians) arm admitted 100% of women who developed preeclampsia within 7 days of the test, whereas the nonreveal (result unknown) arm admitted 83% of women (P=0.038). Use of the sFlt-1/PlGF ratio yielded a sensitivity of 100% (95% CI 85.8 to 100) and a negative predictive value of 100% (95% CI 97.1 to 100) compared with a sensitivity of 83.3% (95% CI 58.6 to 96.4) and negative predictive value of 97.8% (95% CI 93.7 to 99.5) with clinical practice alone. A stratified analysis of the PARROT data also showed that a higher proportion of women with a very low PlGF test result (<12 pg/mL) received prenatal corticosteroids: 38.6% in the revealed testing group compared with 15.8% in the concealed testing group.[59] This may be clinically useful in reducing the rate of respiratory distress syndrome and intraventricular hemorrhage in preterm babies. However, it is unclear whether use of these investigations carries any benefit for perinatal outcomes. Although there was concern that PlGF testing would lead to increased and unnecessary preterm deliveries, the proportion of births before 37 weeks’ gestation in the test and control arms were the same in both PARROT and INSPIRE trials. NICE recommends against using PlGF-based testing to make decisions about whether to offer a planned early birth in women with preterm preeclampsia, which should be based on clinical assessment.[16][55]
Although the Food and Drug Administration (FDA) has approved the sFlt-1/PlGF test to aid risk assessment for the progression to preeclampsia with severe features, the American College of Obstetricians and Gynecologists does not recommend any single biomarker test (e.g., PlGF testing or the sFlt-1/PlGF ratio) for the prediction of preeclampsia, diagnosis or exclusion of preeclampsia with severe features, or determination of the management approach after a positive or negative test result.[1][60]
How to take a venous blood sample from the antecubital fossa using a vacuum needle.
Use of this content is subject to our disclaimer