Often patients with mitral valve prolapse (MVP) are asymptomatic, and auscultatory findings prompt further evaluation. Expert physical exam alone is highly sensitive for MVP, but specificity is limited. Diagnosis is typically established by echocardiography.[1]Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Feb 2;143(5):e72-e227.
https://www.doi.org/10.1161/CIR.0000000000000923
http://www.ncbi.nlm.nih.gov/pubmed/33332150?tool=bestpractice.com
[2]Bonow RO, O'Gara PT, Adams DH, et al. 2020 Focused update of the 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020 May 5;75(17):2236-70.
https://www.doi.org/10.1016/j.jacc.2020.02.005
http://www.ncbi.nlm.nih.gov/pubmed/32068084?tool=bestpractice.com
[3]Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017 Apr;30(4):303-71.
https://www.doi.org/10.1016/j.echo.2017.01.007
http://www.ncbi.nlm.nih.gov/pubmed/28314623?tool=bestpractice.com
Initial clinical evaluation
The classic finding on cardiac auscultation is a midsystolic click, followed by a medium- to high-pitched, late-systolic murmur of varying duration. The click is generated by the tensing of the mitral apparatus as the leaflets prolapse. The murmur is caused by mitral regurgitation (MR). The typical mid- to late-systolic click and murmur of MVP is distinct from the holosystolic murmur of MR resulting from torn chordae tendineae.
Dynamic maneuvers can be used to alter the timing of the click during systole.
With a decrease in preload (standing, Valsalva) or decrease in afterload (amyl nitrate administration), the click and murmur occur earlier in systole. Conversely, with an increase in left ventricular (LV) volume (squatting) or increase in afterload (handgrip) the click and murmur occur later.
Echocardiogram
When clinical exam suggests MVP, an echocardiogram is indicated. This establishes the diagnosis by detecting a leaflet prolapse of ≥2 mm above the level of the annulus during systole in the long-axis parasternal view. It also assesses additional important features such as the presence of left-sided chamber enlargement, leaflet morphology, and the presence and severity of MR.[1]Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Feb 2;143(5):e72-e227.
https://www.doi.org/10.1161/CIR.0000000000000923
http://www.ncbi.nlm.nih.gov/pubmed/33332150?tool=bestpractice.com
[2]Bonow RO, O'Gara PT, Adams DH, et al. 2020 Focused update of the 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020 May 5;75(17):2236-70.
https://www.doi.org/10.1016/j.jacc.2020.02.005
http://www.ncbi.nlm.nih.gov/pubmed/32068084?tool=bestpractice.com
[3]Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017 Apr;30(4):303-71.
https://www.doi.org/10.1016/j.echo.2017.01.007
http://www.ncbi.nlm.nih.gov/pubmed/28314623?tool=bestpractice.com
Mitral annular disjunction (MAD) can be diagnosed by noting the presence of a gap between the base of the posterior mitral leaflet and the LV myocardium, also called “atrialization” of the posterior leaflet. Chronic, hemodynamically significant MR can cause pulmonary hypertension.[26]Van der Bijl P, Stassen J, Haugaa KH, et al. Mitral annular disjunction in the context of mitral valve prolapse: identifying the at-risk patient. JACC Cardiovasc Imaging. 2024 Apr 18:S1936-878X(24)00119-0.
https://www.sciencedirect.com/science/article/pii/S1936878X24001190?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/38703174?tool=bestpractice.com
The effect of chronic MR on LV systolic function can be latent, and ejection fraction can remain in the normal range when contractility is already impaired.[23]Griffin BP. Timing of surgical intervention in chronic mitral regurgitation: is vigilance enough? Circulation. 2006 May 9;113(18):2169-72.
http://circ.ahajournals.org/cgi/content/full/113/18/2169
http://www.ncbi.nlm.nih.gov/pubmed/16684872?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Parasternal long-axis echocardiogram of mitral valve illustrating prolapse of posterior leaflet (red arrow)From the personal collection of Brian Griffin, MD; used with permission [Citation ends].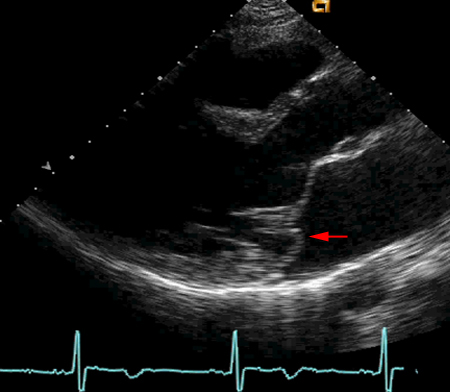
Palpitations or syncope
The correlation between palpitations and MVP is complex and incompletely understood. MVP is found to be associated with malignant ventricular arrhythmias and sudden cardiac arrest in up to 4% of individuals. Myocardial fibrosis and presence of MAD are found to be additional risk markers for ventricular arrhythmia.[28]Aabel EW, Chivulescu M, Lie ØH, et al. Ventricular arrhythmias in arrhythmic mitral valve syndrome-a prospective continuous long-term cardiac monitoring study. Europace. 2023 Feb 16;25(2):506-16.
https://academic.oup.com/europace/article/25/2/506/6763079
http://www.ncbi.nlm.nih.gov/pubmed/36256597?tool=bestpractice.com
Evaluation of palpitations or syncope should mirror the workup in patients without MVP, including evaluation with Holter or ambulatory event monitoring to rule out significant arrhythmias.
Cardiac magnetic resonance imaging (MRI)
Cardiac MRI is an option for patients with unsatisfactory echocardiogram or marked echocardiographic discrepancy with clinical findings. Cardiac MRI adds information about underlying mechanisms, providing accurate quantification of regurgitation, chamber volumes, and myocardial viability. Delayed enhancement in papillary muscle and basal inferior wall may indicate increased arrhythmia risk. LV fibrosis on cardiac MRI is associated with cardiac events including heart failure, arrhythmia, and death.[3]Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017 Apr;30(4):303-71.
https://www.doi.org/10.1016/j.echo.2017.01.007
http://www.ncbi.nlm.nih.gov/pubmed/28314623?tool=bestpractice.com
[29]Garg P, Swift AJ, Zhong L, et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol. 2020 May;17(5):298-312.
https://www.doi.org/10.1038/s41569-019-0305-z
http://www.ncbi.nlm.nih.gov/pubmed/31819230?tool=bestpractice.com
[30]Essayagh B, Iacuzio L, Civaia F, et al. Usefulness of 3-tesla cardiac magnetic resonance to detect mitral annular disjunction in patients with mitral valve prolapse. Am J Cardiol. 2019 Dec 1;124(11):1725-30.
http://www.ncbi.nlm.nih.gov/pubmed/31606191?tool=bestpractice.com
Cardiac MRI and positron emission tomography-computed tomography are recommended for further evaluation of patients with MVP when infective endocarditis is suspected, providing detailed visualization of cardiac structures, and detection of inflammation or infection.[31]Delgado V, Ajmone Marsan N, de Waha S, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. 2023 Oct 14;44(39):3948-4042.
https://academic.oup.com/eurheartj/article/44/39/3948/7243107
