Etiology
The exact etiology of mitral valve prolapse (MVP) is unknown. A genetic link has been identified in familial variants of MVP, including chromosomal abnormalities such as MMVP1 (chromosome 16p), MMVP2 (chromosome 11p15), and MMVP3 (chromosome 13q31).[14][16][17]
Primary MVP is associated with characteristic histologic changes of myxomatous degeneration. These include proliferation of the spongiosa layer (middle layer) of leaflet tissue due to abnormal glycosaminoglycan production, abnormal leaflet collagen structure, and abnormally weak and stretchy leaflet chordae.[14][15][18][19]
MVP with characteristic myxomatous degeneration occurs more frequently in certain connective tissue diseases such as Marfan syndrome, Ehlers-Danlos syndrome, osteogenesis imperfecta, and pseudoxanthoma elasticum.[20][21][22] This suggests that primary MVP may be a related connective tissue disorder.
Pathophysiology
Classic (primary) MVP
In classic MVP, progressive myxomatous changes in leaflets result in mitral regurgitation that may worsen over time. MVP-related mitral regurgitation produces volume overload of the left ventricle (LV) and left atrium. Preload increases and the LV dilates to maintain a normal forward flow. However, the increase in afterload resulting from LV dilation is offset by the ventricle pumping much of its volume, including regurgitant volume, into a low-impedance circuit, the left atrium. Therefore, afterload may be variably reduced initially in mitral regurgitation and typically only becomes elevated in later stages of the disease as LV size increases further. Thus, ejection indices such as ejection fraction are not considered reliable measures of LV contractile function and remain in the normal range when contractility is already impaired.[23] Chordal elongation and leaflet prolapse often result in chordal rupture with prompt deterioration in mitral regurgitation due to flail leaflet and loss of coaptation.
Nonclassic (secondary or functional) MVP
Secondary or functional MVP occurs when histologically normal valves prolapse. This occurs due to an imbalance of geometric features that normally govern mitral valve mechanical function such as LV size, mitral annular dimensions, and mitral leaflet size. For example, in younger women who are volume depleted, a disproportionately small LV cavity dimension may result in prolapse.[24] Another example is the occurrence of MVP associated with secundum atrial septal defects.[25]
Mitral regurgitation
Mitral regurgitation occurs in MVP either due to progressive myxomatous degeneration or due to chordal rupture with resultant flail segment. Progression of mitral regurgitation can be abrupt when related to chordal rupture.
Classification
Histology-based classification
Classic or primary MVP involves histologic changes in the valve leaflet. Nonclassic MVP, also termed secondary or functional MVP, is the prolapse of histologically normal valves.
Classic (primary)
Leaflet thickening of 5 mm or greater during diastasis, reflecting myxomatous degeneration. These changes are predictors of complications.[5][6]
Also known as Barlow’s disease and characterized by thickened and elongated leaflets with multi-segment prolapse, dilated annulus (>36 mm), and elongated chords. This phenotype is most common in younger patients (<60 years at the time of surgery) who manifest with long standing history of mitral regurgitation.[11]
Most often sporadic, but familial variants are recognized.
Familial variants are typically autosomal-dominant inheritance with incomplete penetrance.[10][12]
May be associated with connective tissue such as in Marfan syndrome.
Nonclassic (secondary or functional)
Histologically normal valves prolapse.
Involves mismatch between ventricular size and mitral valve apparatus, associated with a variety of conditions, including atrial septal defect, anorexia, hypertrophic cardiomyopathy, volume depletion, or papillary muscle ischemia.
A well described phenotype, also considered nonclassic MVP, is known as fibroelastic deficiency (FED). It is associated with fibrillin deficit and often leads to rupture of one or more thinned chordae, usually of the posterior medial segment. The leaflets appear completely normal or thinned. The prolapsed segment may develop isolated myxomatous changes with mucopolysaccharide accumulation. This is most commonly encountered in older patients (>60 years at the time of surgery) with a short clinical history. An intermediate phenotype between Barlow’s disease and FED is known as forme fruste, in which the valves have excess tissue and myxomatous changes in one or more segments, but without implying a large valve size.[11]
Leaflet involvement
Disease may be classified based on the number of leaflets involved. Unileaflet disease is more common than bileaflet MVP. In unileaflet MVP, the posterior leaflet is more commonly affected. [Figure caption and citation for the preceding image starts]: Mitral valve en-face view with single P2 segment prolapseReprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2021. All Rights Reserved [Citation ends].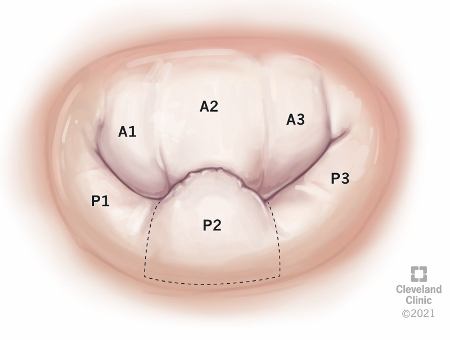
MVP syndrome
Previously, a constellation of findings was associated with MVP and termed MVP syndrome. These included atypical chest pain, anxiety, syncope, palpitations, exercise intolerance, dyspnea, low blood pressure, and abnormal ECG. However, these associations were incorrect and likely reflected poor study design and selection bias in older studies. In the unselected outpatient population studied prospectively in the Framingham Heart Study, the only association found with MVP was low body weight.[13]
Use of this content is subject to our disclaimer