Discuss the treatment strategy with the multidisciplinary team for patients with advanced nasopharyngeal cancer (NPC).[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
For localized or locally advanced NPC, curative-intent radiation therapy, mainly intensity-modulated radiation therapy (IMRT) with daily image guidance, is the main treatment modality.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Concurrent chemotherapy is added for locally advanced disease.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Concurrent chemotherapy can be offered for selective patients with localized disease if there are adverse features such as bulky tumor volumes or high Epstein-Barr virus (EBV) DNA copy number.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
For recurrent/metastatic NPC, potential curative-intent treatment, such as nasopharyngectomy, brachytherapy, radiosurgery, stereotactic radiation therapy, IMRT, or surgery followed by chemoradiation, should be considered first, if feasible.[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
Palliative chemotherapy can be considered for selected patients. For patients with newly diagnosed metastatic NPC, locoregional radiation therapy should be considered for locoregional control.[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
When planning treatment, inquire about a history of prior cancer treatments and any residual toxicity, and any history of connective tissue diseases (these may increase complications from chemotherapy and radiation therapy). Ask the patient about any other drugs they are taking concurrently, including supplements.
Use a scale, such as the Eastern Cooperative Oncology Group (ECOG) performance status scale, to determine the patient’s current level of physical functioning and any support they might need.
OncologyPRO: Performance scales - Karnofsky & ECOG scores
Opens in new window Assessments that are useful to perform as baseline evaluations prior to starting treatment include:[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Audiogram (not required but highly recommended prior to platinum chemotherapy)
Speech and swallowing consultation and formal assessment by modified barium swallow/videofluoroscopy
Dental evaluation and counseling in preparation for radiation therapy
Nutrition evaluation
Screening for hepatitis B
Smoking cessation advice, where necessary
Screen for depression.
Early-stage disease (stage 1-2)
Radiation therapy is the mainstay of treatment for nonmetastatic NPC given that it is a radiosensitive tumor and typically in a location that limits complete surgical resection.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
The goal of treatment is cure for patients with nonmetastatic NPC.[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
Principles of radiation therapy
Conformal external beam radiation therapy (EBRT) techniques, including intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy, are considered the standard of care. Nodal metastases are common in NPC and subclinical radiation therapy targets generally encompass a large anatomical area that includes the retropharyngeal lymph nodes and bilateral II-V lymph node stations. Level IB is electively covered in select patients with a high burden of level II nodal disease or anterior nasal cavity involvement.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
Stage 1 (T1, N0, M0)
For patients with stage 1 disease, the recommended treatment is radiation therapy without concurrent chemotherapy.[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
In patients with clinically and radiographically undetectable nodal metastases (N0), reducing the nodal elective radiation therapy target volume to exclude level IV (low neck) can be considered.[30]Tang LL, Huang CL, Zhang N, et al. Elective upper-neck versus whole-neck irradiation of the uninvolved neck in patients with nasopharyngeal carcinoma: an open-label, non-inferiority, multicentre, randomised phase 3 trial. Lancet Oncol. 2022 Apr;23(4):479-90.
http://www.ncbi.nlm.nih.gov/pubmed/35240053?tool=bestpractice.com
Stage 2 (T2, N0, M0)
Similar to patients with stage 1 disease, for patients with low-risk stage 2 disease (N0 and pretreatment plasma EBV DNA <4000 copies/mL), the recommended treatment is radiation therapy without concurrent chemotherapy, but with concurrent chemotherapy if high-risk features are present (such as bulky tumor volumes or high EBV DNA copy number).[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
In patients with clinically and radiographically undetectable nodal metastases (N0), reducing the nodal elective radiation therapy target volume to exclude level IV (low neck) can be considered.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
Stage 2 (T0 (EBV+)-T2, N1, M0)
Stage 2 disease with retropharyngeal or ipsilateral cervical nodal metastases (N1) is a heterogeneous category and additional high-risk features have been found to be prognostic for poorer outcomes (node ≥3 cm, level IV or VB lymph node [low neck], extranodal extension, pretreatment plasma EBV DNA ≥4000 copies/mL).[31]Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016 Feb 15;122(4):546-58.
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.29795
http://www.ncbi.nlm.nih.gov/pubmed/26588425?tool=bestpractice.com
[32]Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006 Dec 1;24(34):5414-8.
scopubs.org/doi/10.1200/JCO.2006.07.7982?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/17135642?tool=bestpractice.com
[33]Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016 Oct 15;388(10054):1883-92.
http://www.ncbi.nlm.nih.gov/pubmed/27567279?tool=bestpractice.com
National Comprehensive Cancer Network (NCCN) guidelines recommend concurrent chemotherapy and radiation therapy for these patients.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Cisplatin should be considered as the standard concurrent chemotherapy agent.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
For patients who cannot tolerate cisplatin (e.g., if there is preexisting chronic kidney disease or hearing impairment), carboplatin is a reasonable alternative.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
The addition of induction or adjuvant chemotherapy is generally not recommended but could be considered after multidisciplinary discussion in select patients with large tumor burden or very high pretreatment EBV DNA copy number.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
[Figure caption and citation for the preceding image starts]: Lymph node groups in the head and neck; the numbers refer to the anatomic levels of the lymph nodesCancer Research UK [Citation ends].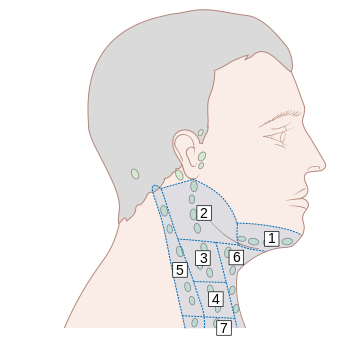
Advanced-stage disease (stage 3 and 4A)
Stage 3 (T3, N0, M0)
NPC with a more extensive primary tumor and without nodal metastases (T3, N0, M0) is a distinct category of advanced stage NPC that has traditionally been treated more akin to stage 1-2 NPC with either radiation therapy alone or concurrent chemoradiation.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Current guidelines vary, though all generally recommend a risk-stratified approach with consideration for high-risk features, such as tumor volume and pretreatment EBV DNA levels.
Guidelines recommend radiation therapy plus concurrent chemotherapy for patients with stage 3 (T3, N0, M0) disease.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
The addition of induction or adjuvant chemotherapy is generally not recommended but could be considered after multidisciplinary discussion in select patients with large tumor burden or very high pretreatment EBV DNA levels.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Stage 3-4A (T3, N1-3, M0; OR T4, N0-3, M0; OR T0 (EBV+)-T2, N2-3, M0)
For all other locally advanced NPC (3-4A, excluding T3, N0, M0), induction chemotherapy followed by concurrent chemoradiation is recommended for the majority of patients.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Gemcitabine plus cisplatin, or docetaxel plus cisplatin plus fluorouracil are the preferred induction regimens.[1]Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021 Mar 1;39(7):840-59.
https://ascopubs.org/doi/10.1200/JCO.20.03237?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33405943?tool=bestpractice.com
[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
[34]Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019 Sep 19;381(12):1124-35.
https://www.nejm.org/doi/10.1056/NEJMoa1905287?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/31150573?tool=bestpractice.com
For patients ineligible for induction chemotherapy, adjuvant chemotherapy with cisplatin plus fluorouracil is recommended after completion of concurrent chemoradiation.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
[35]Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998 Apr;16(4):1310-7.
https://ascopubs.org/doi/10.1200/JCO.1998.16.4.1310?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/9552031?tool=bestpractice.com
Stage 3-4A (excluding T3, N0, M0) NPC is a particularly heterogeneous category. As such, participation in clinical trials is especially encouraged to better select subgroups of patients for more intensive treatments and other subgroups for less intensive treatments.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Metastatic disease
The preferred first-line treatment for patients with metastatic NPC who have no surgery or radiation therapy options is toripalimab (a programmed cell death protein 1 [PD-1] inhibitor) plus cisplatin plus gemcitabine.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Other recommended first-line options include:[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
cisplatin plus gemcitabine
cisplatin plus gemcitabine plus tislelizumab
cisplatin plus gemcitabine plus pembrolizumab or nivolumab
cisplatin plus fluorouracil
cisplatin or carboplatin plus docetaxel or paclitaxel
carboplatin plus cetuximab
gemcitabine plus carboplatin.
Locoregional nasopharyngeal and/or neck nodal recurrence
Salvage nasopharyngectomy and/or neck dissection should be considered for small locoregional recurrences amenable for surgical resection.[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
Radical, modified radical, or selective neck dissection can be used for nodal neck recurrence.[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
The NCCN classifies cervical lymphadenectomy as either comprehensive or selective.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers [internet publication].
https://www.nccn.org/guidelines/category_1
Adjuvant radiation or chemoradiation is frequently offered after salvage surgery. There are limited data supporting the practice, but reradiation therapy after salvage surgery is supported by consensus guidelines.[36]Ng WT, Soong YL, Ahn YC, et al. International recommendations on reirradiation by intensity modulated radiation therapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2021 Jul 1;110(3):682-95.
http://www.ncbi.nlm.nih.gov/pubmed/33571626?tool=bestpractice.com
For unresectable locoregional recurrence, reradiation therapy should be considered before a chemotherapy-only approach. Reradiation therapy is challenging since many organs at risk have already been exposed to near maximal safe doses of radiation from the first course of radiation therapy and careful patient selection is needed.[2]Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Apr;32(4):452-65.
https://www.annalsofoncology.org/article/S0923-7534(20)43210-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33358989?tool=bestpractice.com
International recommendations generally favor reradiation therapy after at least 12 months latency between courses of radiation therapy to allow normal tissues to recover from the initial course of radiation therapy.[36]Ng WT, Soong YL, Ahn YC, et al. International recommendations on reirradiation by intensity modulated radiation therapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2021 Jul 1;110(3):682-95.
http://www.ncbi.nlm.nih.gov/pubmed/33571626?tool=bestpractice.com
Unlike the first course of radiation therapy that includes targeting subclinical disease, reradiation therapy targets gross recurrent tumor only. For bulky recurrences, induction with concurrent chemotherapy is favored among experts with reradiation therapy.
IMRT/volumetric modulated arc therapy (VMAT) is viewed as an appropriate modality for reradiation therapy, although if particle therapy, such as proton therapy, is available it could be considered as well and may be preferred for select recurrences.[36]Ng WT, Soong YL, Ahn YC, et al. International recommendations on reirradiation by intensity modulated radiation therapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2021 Jul 1;110(3):682-95.
http://www.ncbi.nlm.nih.gov/pubmed/33571626?tool=bestpractice.com
Locoregional recurrence not amenable for salvage surgery or reradiation therapy is treated with palliative chemotherapy.
