Acute lymphoblastic leukemia (ALL) commonly presents rapidly and aggressively in adults. It may mimic many other conditions, which often creates diagnostic confusion.
A definitive diagnosis can be made from a bone marrow aspiration and trephine biopsy. Leukemic lymphoblasts may circulate in the blood; if present in sufficient numbers, this may be used to defer bone marrow exam at presentation.
History
The appearance of signs and symptoms associated with cytopenias is often the initial cause for seeking medical attention by the patient. Most patients present within a few weeks of symptom onset.
Anemia typically manifests as fatigue, dyspnea, palpitations, and dizziness; thrombocytopenia presents with bleeding (e.g., epistaxis, menorrhagia) and easy bruising; neutropenia presents as recurrent infections, which may cause fever.
Lymph node involvement is common in ALL. Enlarged lymph nodes can be an initial presenting cause.
Abdominal and bone pain may be present due to infiltration of blast cells in the spleen and bone marrow, respectively.[47]Gallagher DJ, Phillips DJ, Heinrich SD. Orthopedic manifestations of acute pediatric leukemia. Orthop Clin North Am. 1996 Jul;27(3):635-44.
http://www.ncbi.nlm.nih.gov/pubmed/8649744?tool=bestpractice.com
Historic factors suggestive of ALL include history of malignancy, family history of ALL, genetic disorder (e.g., trisomy 21, Klinefelter syndrome, Fanconi anemia, Bloom syndrome, ataxia-telangiectasia), treatment with chemotherapy, radiation exposure, and smoking.[1]Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004 Apr 8;350(15):1535-48.
http://www.ncbi.nlm.nih.gov/pubmed/15071128?tool=bestpractice.com
[2]Hoelzer D, Gökbuget N, Ottmann O, et al. Acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2002 Jan;(1):162-92.
https://ashpublications.org/hematology/article/2002/1/162/18610/Acute-Lymphoblastic-Leukemia
http://www.ncbi.nlm.nih.gov/pubmed/12446423?tool=bestpractice.com
[3]Jabbour EJ, Faderl S, Kantarjian HM. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2005 Nov;80(11):1517-27.
http://www.ncbi.nlm.nih.gov/pubmed/16295033?tool=bestpractice.com
[11]Greaves MF, Maia AT, Wiemels JL, et al. Leukemia in twins: lessons in natural history. Blood. 2003 Oct 1;102(7):2321-33.
http://www.ncbi.nlm.nih.gov/pubmed/12791663?tool=bestpractice.com
[12]De Keersmaecker K, Marynen P, Cools J. Genetic insights in the pathogenesis of T-cell acute lymphoblastic leukemia. Haematologica. 2005 Aug;90(8):1116-27.
http://www.ncbi.nlm.nih.gov/pubmed/16079112?tool=bestpractice.com
[13]Machatschek JN, Schrauder A, Helm F, et al. Acute lymphoblastic leukemia and Klinefelter syndrome in children: two cases and review of the literature. Pediatr Hematol Oncol. 2004 Oct-Nov;21(7):621-6.
http://www.ncbi.nlm.nih.gov/pubmed/15626018?tool=bestpractice.com
[14]Bloom M, Maciaszek JL, Clark ME, et al. Recent advances in genetic predisposition to pediatric acute lymphoblastic leukemia. Expert Rev Hematol. 2020 Jan;13(1):55-70.
http://www.ncbi.nlm.nih.gov/pubmed/31657974?tool=bestpractice.com
[19]Snyder DS, Stein AS, O'Donnell MR, et al, Philadelphia chromosome-positive acute lymphoblastic leukemia secondary to chemoradiotherapy for Ewing sarcoma. Report of two cases and concise review of the literature. Am J Hematol. 2005 Jan;78(1):74-8.
http://www.ncbi.nlm.nih.gov/pubmed/15609284?tool=bestpractice.com
[30]Hoffman R, Shattil SJ, Furie B, et al. Hematology: basic principles and practice. Vol 1. 4th ed. Orlando, FL: Churchill Livingstone / W.B. Saunders; 2005.[32]Faderl S, Jeha S, Kantarjian HM. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003 Oct 1;98(7):1337-54.
http://www.ncbi.nlm.nih.gov/pubmed/14508819?tool=bestpractice.com
[48]Abeloff MD, Armitage J, Niederhuber JE, et al. Clinical oncology. 3rd ed. Vol 1. Philadelphia, PA: Churchill Livingstone; 2004:3232.
Less common ALL presentations
These include eosinophilia, isolated renal failure, pulmonary nodules, bone marrow necrosis, pleural/pericardial effusion, superior vena cava obstruction, hypoglycemia, joint pain, mental status changes, headaches, chin numbness, and skin nodules.[1]Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004 Apr 8;350(15):1535-48.
http://www.ncbi.nlm.nih.gov/pubmed/15071128?tool=bestpractice.com
[8]Haferlach T, Bacher U, Kern W, et al. Diagnostic pathways in acute leukemias: a proposal for a multimodal approach. Ann Hematol. 2007 May;86(5):311-27.
http://www.ncbi.nlm.nih.gov/pubmed/17375301?tool=bestpractice.com
Physical examination
Findings may include pallor, ecchymoses, petechiae, lymphadenopathy, hepatosplenomegaly, mediastinal masses, abdominal masses, testicular enlargement (unilateral and painless), renal enlargement, and skin infiltrations (skin nodules).
Lymphadenopathy is classically generalized, and the enlarged nodes are painless and freely movable.[1]Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004 Apr 8;350(15):1535-48.
http://www.ncbi.nlm.nih.gov/pubmed/15071128?tool=bestpractice.com
[8]Haferlach T, Bacher U, Kern W, et al. Diagnostic pathways in acute leukemias: a proposal for a multimodal approach. Ann Hematol. 2007 May;86(5):311-27.
http://www.ncbi.nlm.nih.gov/pubmed/17375301?tool=bestpractice.com
T-ALL more commonly causes mediastinal masses, whereas B-ALL more commonly causes abdominal masses. The findings of stridor, wheezing, pericardial effusion, and superior vena cava syndrome may be associated with mediastinal masses. Mature B-ALL (Burkitt lymphoma/leukemia) may initially present as a palpable large abdominal mass from a rapidly proliferating tumor.[30]Hoffman R, Shattil SJ, Furie B, et al. Hematology: basic principles and practice. Vol 1. 4th ed. Orlando, FL: Churchill Livingstone / W.B. Saunders; 2005.[48]Abeloff MD, Armitage J, Niederhuber JE, et al. Clinical oncology. 3rd ed. Vol 1. Philadelphia, PA: Churchill Livingstone; 2004:3232.
Testicular involvement occurs most commonly in children and adolescents with T-ALL.[49]Nguyen HTK, Terao MA, Green DM, et al. Testicular involvement of acute lymphoblastic leukemia in children and adolescents: diagnosis, biology, and management. Cancer. 2021 Sep 1;127(17):3067-81.
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.33609
http://www.ncbi.nlm.nih.gov/pubmed/34031876?tool=bestpractice.com
Testicular examination should be carried out at diagnosis in all male patients. The testes can represent a sanctuary site that is relatively protected from the effects of systemic therapy via the blood-testis barrier.[49]Nguyen HTK, Terao MA, Green DM, et al. Testicular involvement of acute lymphoblastic leukemia in children and adolescents: diagnosis, biology, and management. Cancer. 2021 Sep 1;127(17):3067-81.
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.33609
http://www.ncbi.nlm.nih.gov/pubmed/34031876?tool=bestpractice.com
Neurologic assessment
Required to exclude central nervous system (CNS) involvement, which is a major complication of ALL. CNS involvement occurs in approximately 5% to 7% of patients at diagnosis; incidence is highest in patients with T-ALL (8%) and mature B-ALL (Burkitt lymphoma/leukemia, 13%).[50]Reman O, Pigneux A, Huguet F, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis and/or at first relapse: results from the GET-LALA group. Leuk Res. 2008 Nov;32(11):1741-50.
http://www.ncbi.nlm.nih.gov/pubmed/18508120?tool=bestpractice.com
[51]Lazarus HM, Richards SM, Chopra R, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 2006 Jul 15;108(2):465-72.
https://ashpublications.org/blood/article/108/2/465/109893/Central-nervous-system-involvement-in-adult-acute
http://www.ncbi.nlm.nih.gov/pubmed/16556888?tool=bestpractice.com
[52]Pui CH. Central nervous system disease in acute lymphoblastic leukemia: prophylaxis and treatment. Hematology Am Soc Hematol Educ Program. 2006 Jan;(1):142-6.
https://ashpublications.org/hematology/article/2006/1/142/19688/Central-Nervous-System-Disease-in-Acute
http://www.ncbi.nlm.nih.gov/pubmed/17124053?tool=bestpractice.com
[53]Lamanna N, Weiss M. Treatment options for newly diagnosed patients with adult acute lymphoblastic leukemia. Curr Hematol Rep. 2004 Jan;3(1):40-6.
http://www.ncbi.nlm.nih.gov/pubmed/14695849?tool=bestpractice.com
[54]Richards S, Pui CH, Gayon P, et al. Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013 Feb;60(2):185-95.
https://onlinelibrary.wiley.com/doi/10.1002/pbc.24228
http://www.ncbi.nlm.nih.gov/pubmed/22693038?tool=bestpractice.com
The meninges are the primary site of CNS disease.[55]Frishman-Levy L, Izraeli S. Advances in understanding the pathogenesis of CNS acute lymphoblastic leukaemia and potential for therapy. Br J Haematol. 2017 Jan;176(2):157-67.
https://onlinelibrary.wiley.com/doi/10.1111/bjh.14411
http://www.ncbi.nlm.nih.gov/pubmed/27766623?tool=bestpractice.com
Presenting features of CNS disease include focal neurologic signs, headache, papilledema, nuchal rigidity, and meningismus. Some patients may present with signs or symptoms of focal neurologic deficit (e.g., diplopia) due to isolated sites of CNS involvement on the cranial nerves (mainly the seventh, third, fourth, and sixth).[52]Pui CH. Central nervous system disease in acute lymphoblastic leukemia: prophylaxis and treatment. Hematology Am Soc Hematol Educ Program. 2006 Jan;(1):142-6.
https://ashpublications.org/hematology/article/2006/1/142/19688/Central-Nervous-System-Disease-in-Acute
http://www.ncbi.nlm.nih.gov/pubmed/17124053?tool=bestpractice.com
Initial laboratory tests
Should include complete blood count with differential, peripheral blood smear, comprehensive metabolic panel (serum electrolytes; serum uric acid; serum lactate dehydrogenase [LDH]; renal function tests; liver function tests), coagulation profile (prothrombin time, partial thromboplastin time, fibrinogen, and D-dimers), blood type and antibody screening for eventual transfusion support, and viral antibody testing (including cytomegalovirus, hepatitis B and C, and HIV serologies).
Laboratory findings
Over 90% of patients have clinically evident hematologic abnormalities at the time of initial diagnosis. Normocytic normochromic anemia with low reticulocyte count is present in 80% of patients. Thrombocytopenia is very common, affecting 75% of patients.[1]Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004 Apr 8;350(15):1535-48.
http://www.ncbi.nlm.nih.gov/pubmed/15071128?tool=bestpractice.com
[2]Hoelzer D, Gökbuget N, Ottmann O, et al. Acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2002 Jan;(1):162-92.
https://ashpublications.org/hematology/article/2002/1/162/18610/Acute-Lymphoblastic-Leukemia
http://www.ncbi.nlm.nih.gov/pubmed/12446423?tool=bestpractice.com
[8]Haferlach T, Bacher U, Kern W, et al. Diagnostic pathways in acute leukemias: a proposal for a multimodal approach. Ann Hematol. 2007 May;86(5):311-27.
http://www.ncbi.nlm.nih.gov/pubmed/17375301?tool=bestpractice.com
Leukocytosis is found in 50% of patients. In 25% of these patients, white blood cell (WBC) count is >50,000/microliter. High WBC at presentation is associated with a poorer prognosis. Despite the elevation in WBC, many patients have severe neutropenia (absolute neutrophil count <500 cells/microliter), thus placing them at high risk for serious infections.[56]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prevention and treatment of cancer-related infections [internet publication].
https://www.nccn.org/guidelines/category_3
See Febrile neutropenia.
Leukemic lymphoblasts may be detected on peripheral blood smear. The presence of leukemic lymphoblasts ≥1000/microliter in the peripheral blood is sufficient to defer bone marrow exam at presentation.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
Hypercalcemia may occur due to bony infiltration or ectopic release of a parathyroid hormone-like substance.
Hyperkalemia, hyperphosphatemia, hyperuricemia, hypocalcemia, and elevated serum LDH may occur due to tumor lysis syndrome (TLS), particularly during treatment and if WBC count (tumor burden) is high. This can lead to cardiac arrhythmias, seizures, acute renal failure, and death, if untreated. TLS is an oncologic emergency. See Tumor lysis syndrome.
Evaluation of bone marrow
The diagnostic workup for ALL requires evaluation of bone marrow aspirate and trephine biopsy specimens (or peripheral blood if sufficient numbers of circulating lymphoblasts are present) using cytomorphology assessment, immunophenotyping, molecular studies, and cytogenetic analysis.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[58]de Haas V, Ismaila N, Advani A, et al. Initial diagnostic work-up of acute leukemia: ASCO clinical practice guideline endorsement of the College of American Pathologists and American Society of Hematology guideline. J Clin Oncol. 2019 Jan 20;37(3):239-53.
https://ascopubs.org/doi/10.1200/JCO.18.01468
http://www.ncbi.nlm.nih.gov/pubmed/30523709?tool=bestpractice.com
These investigations are important for risk stratification, treatment planning, and establishing a baseline for measurable residual disease (MRD) assessment during treatment.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
Hematopathology evaluation
Bone marrow aspiration specimens should be stained with Wright-Giemsa stain, and trephine biopsy specimens should be stained with hematoxylin and eosin. Biopsy specimens should also be stained with myeloperoxidase, which will be negative in patients with ALL.[59]Hoelzer D, Bassan R, Dombret H, et al. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016 Sep;27(suppl 5):v69-82.
https://www.annalsofoncology.org/article/S0923-7534(19)31639-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27056999?tool=bestpractice.com
A biopsy demonstrating bone marrow hypercellularity and infiltration by lymphoblasts is characteristic for ALL. There is a lack of consensus regarding the proportion of lymphoblasts in the bone marrow that is required to make a diagnosis of ALL; however, a threshold of ≥20% is generally advised.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
The proportion of lymphoblasts in the bone marrow can help distinguish between ALL and lymphoblastic lymphoma. See Differential diagnosis. A defined number of lymphoblasts in the bone marrow (or peripheral blood) is not always required for the diagnosis of ALL (e.g., T-ALL can be diagnosed based on immunophenotyping). See Classification.
Immunophenotypic findings
Immunophenotyping (on bone marrow specimens, or peripheral blood if sufficient numbers of circulating lymphoblasts are present) using flow cytometry is required to assess cell surface markers to:[1]Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004 Apr 8;350(15):1535-48.
http://www.ncbi.nlm.nih.gov/pubmed/15071128?tool=bestpractice.com
[3]Jabbour EJ, Faderl S, Kantarjian HM. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2005 Nov;80(11):1517-27.
http://www.ncbi.nlm.nih.gov/pubmed/16295033?tool=bestpractice.com
[8]Haferlach T, Bacher U, Kern W, et al. Diagnostic pathways in acute leukemias: a proposal for a multimodal approach. Ann Hematol. 2007 May;86(5):311-27.
http://www.ncbi.nlm.nih.gov/pubmed/17375301?tool=bestpractice.com
determine lymphoid lineage (B-cell or T-cell);
define an aberrant phenotype for MRD assessment; and
detect clinically important cell surface antigens (e.g., CD20).
Leukemic cells typically exhibit markers of one cell type. Rarely, simultaneous expression of lymphoid and myeloid markers occurs in ALL, either as ALL with aberrant expression of myeloid antigens (My+ ALL) or true biphenotypic acute leukemia.
Molecular and cytogenetic evaluation
Molecular studies (e.g., reverse transcriptase polymerase chain reaction [RT-PCR]) and cytogenetic analysis (e.g., karyotyping, fluorescence in situ hybridization [FISH]) are required to detect genetic abnormalities (e.g., BCR::ABL1 fusion gene encoded by the Philadelphia chromosome; ETV6::RUNX1 [also known as TEL::AML1]; KMT2A rearrangement). Findings may be of prognostic and therapeutic significance.[58]de Haas V, Ismaila N, Advani A, et al. Initial diagnostic work-up of acute leukemia: ASCO clinical practice guideline endorsement of the College of American Pathologists and American Society of Hematology guideline. J Clin Oncol. 2019 Jan 20;37(3):239-53.
https://ascopubs.org/doi/10.1200/JCO.18.01468
http://www.ncbi.nlm.nih.gov/pubmed/30523709?tool=bestpractice.com
Next-generation sequencing assays can be used to detect certain gene fusions and mutations with prognostic significance (e.g., NOTCH1/FBXW7 mutation in T-ALL).[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[59]Hoelzer D, Bassan R, Dombret H, et al. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016 Sep;27(suppl 5):v69-82.
https://www.annalsofoncology.org/article/S0923-7534(19)31639-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27056999?tool=bestpractice.com
See Diagnostic criteria.
[Figure caption and citation for the preceding image starts]: Lymphoblasts in bone marrow smear from 3-year-old male with ALL (Wright-Giemsa stain)Image and description are from the AFIP Atlas of Tumor Pathology [Citation ends].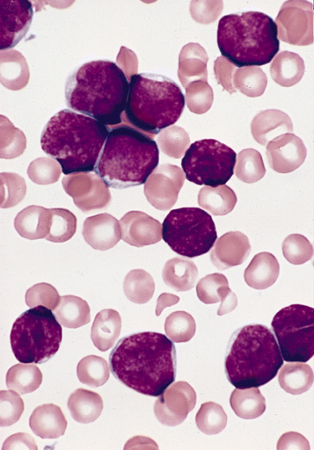
Baseline measurable residual disease (MRD) testing
It is important to establish a baseline for MRD testing based on immunophenotypic, molecular, and/or cytogenetic features of the leukemic cell. MRD testing enables depth and speed of remission to be assessed during treatment. It is prognostically important and can guide therapeutic decisions. The exact tests for MRD depend on the patient, and assays available to the treating center.
The preferred sample for MRD testing is the first small volume (of up to 3 mL) pull of the bone marrow aspirate and/or peripheral blood.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
Lumbar puncture
A lumbar puncture is required in all patients given the relatively high frequency of CNS involvement. Signs or symptoms of CNS involvement include focal neurologic deficits, headache, and meningismus. The procedure should only be performed once raised intracranial pressure has been ruled out.
Lumbar puncture should be carried out at a time consistent with the treatment protocol being used. Pediatric-inspired protocols typically include lumbar puncture at diagnostic workup. However, the National Comprehensive Cancer Network ALL Panel recommends the first lumbar puncture be performed concomitantly with initial intrathecal therapy to avoid seeding the CNS with circulating leukemic blasts, unless symptoms require a lumbar puncture to be performed earlier.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
Detection of lymphoblasts in the initial cerebrospinal fluid (CSF) sample by multiparameter flow cytometry can identify patients at high risk of CNS relapse.[66]Del Principe MI, Buzzatti E, Piciocchi A, et al. Clinical significance of occult central nervous system disease in adult acute lymphoblastic leukemia. A multicenter report from the Campus ALL Network. Haematologica. 2021 Jan 1;106(1):39-45.
https://haematologica.org/article/view/9587
http://www.ncbi.nlm.nih.gov/pubmed/31879328?tool=bestpractice.com
[67]Garcia KA, Cherian S, Stevenson PA, et al. Cerebrospinal fluid flow cytometry and risk of central nervous system relapse after hyperCVAD in adults with acute lymphoblastic leukemia. Cancer. 2022 Apr 1;128(7):1411-7.
http://www.ncbi.nlm.nih.gov/pubmed/34931301?tool=bestpractice.com
CNS involvement at diagnosis can be graded based on the presence of lymphoblasts, WBCs, and red blood cells (RBCs) in the CSF, using the Children’s Oncology Group classification.[68]Winick N, Devidas M, Chen S, et al. Impact of initial CSF findings on outcome among patients with National Cancer Institute standard- and high-risk B-cell acute lymphoblastic leukemia: a report from the children's oncology group. J Clin Oncol. 2017 Aug 1;35(22):2527-34.
https://ascopubs.org/doi/10.1200/JCO.2016.71.4774
http://www.ncbi.nlm.nih.gov/pubmed/28535084?tool=bestpractice.com
[69]Kopmar NE, Cassaday RD. How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood. 2023 Mar 23;141(12):1379-88.
https://ashpublications.org/blood/article/141/12/1379/493851/How-I-prevent-and-treat-central-nervous-system
http://www.ncbi.nlm.nih.gov/pubmed/36548957?tool=bestpractice.com
Higher grade (i.e., increased lymphoblasts, WBCs, and RBCs [traumatic lumbar puncture] in the CSF) is associated with poorer outcomes. See Diagnostic criteria.
Imaging
Chest radiograph may be performed to identify a mediastinal mass, pleural effusion, or lower respiratory tract infection. Pleural effusions should be tapped and samples sent for cytology and immunophenotyping. A mediastinal biopsy should be avoided if possible, though this may be the primary site of involvement for some patients and in such cases is unavoidable.
CNS imaging (e.g., computed tomography [CT]/magnetic resonance imaging [MRI] brain) should be performed in patients with major neurologic signs and symptoms (e.g., lowered consciousness level, meningismus, or focal neurologic deficits) to identify meningeal involvement or CNS bleeding. Spinal cord and parenchymal brain involvement may occur, but is very rare.
CT thorax should be performed in the presence of a widened mediastinum on chest radiograph. CT thorax, abdomen, and pelvis should be performed if there is palpable lymphadenopathy or other evidence of extramedullary disease.
In males with an abnormal testicular exam or symptoms, scrotal ultrasound should be performed to characterize the nature of the abnormality and to establish a baseline prior to treatment initiation.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
Echocardiogram or multigated acquisition (MUGA) scan should be considered in all patients to assess cardiac function before initiating treatment.[57]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
Anthracyclines are used in most treatment regimens for ALL and are potentially cardiotoxic.[Figure caption and citation for the preceding image starts]: Chest x-ray of patient presenting with dyspnea, showing widened mediastinum and tracheal displacementFrom the personal collection of CR Kelsey [Citation ends].
Other investigations
The following tests may be carried out once a diagnosis of ALL is confirmed:[2]Hoelzer D, Gökbuget N, Ottmann O, et al. Acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2002 Jan;(1):162-92.
https://ashpublications.org/hematology/article/2002/1/162/18610/Acute-Lymphoblastic-Leukemia
http://www.ncbi.nlm.nih.gov/pubmed/12446423?tool=bestpractice.com
[3]Jabbour EJ, Faderl S, Kantarjian HM. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2005 Nov;80(11):1517-27.
http://www.ncbi.nlm.nih.gov/pubmed/16295033?tool=bestpractice.com
[70]Relling MV, Schwab M, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019 May;105(5):1095-105.
https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.1304
http://www.ncbi.nlm.nih.gov/pubmed/30447069?tool=bestpractice.com
Human leukocyte antigen (HLA)-typing
Thiopurine methyltransferase (TPMT) phenotyping
Nudix hydrolase 15 (NUDT15) phenotyping
HLA-typing is required to identify a suitable donor for stem cell transplantation and for obtaining HLA-matched platelets in the event of platelet alloimmunization during platelet transfusion.
TPMT and NUDT15 phenotyping help to guide dosing of mercaptopurine during maintenance therapy.[70]Relling MV, Schwab M, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019 May;105(5):1095-105.
https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.1304
http://www.ncbi.nlm.nih.gov/pubmed/30447069?tool=bestpractice.com


