Approach
Your Organizational Guidance
ebpracticenet urges you to prioritize the following organizational guidance:
Diabetes Mellitus Type 2Published by: Domus Medica | SSMGLast published: 2017Diabète sucré de type 2Published by: SSMG | Domus MedicaLast published: 2017Type 2 diabetes is most often diagnosed on routine screening. Strong risk factors, which also indicate the need for screening, include: older age; overweight/obesity; African-American, Latino, Native American, Asian-American, or Pacific Islander ancestry; first-degree relative with diabetes; history of gestational diabetes; presence of prediabetes; physical inactivity; sedentary lifestyle; polycystic ovary syndrome; hypertension; dyslipidemia; known cardiovascular disease; or other clinical conditions associated with insulin resistance (e.g., acanthosis nigricans).[2] Screening should also be considered in people on certain drugs, such as corticosteroids, statins, thiazide diuretics, some HIV antiretrovirals, and second-generation antipsychotics, as these agents are known to increase the risk of diabetes.[2] If results are normal, the American Diabetes Association (ADA) recommends that testing should be repeated at least every 3 years, with consideration of more frequent testing depending on initial results and risk status. People with prediabetes (hemoglobin A1c [HbA1c] 5.7% to 6.4% [39-47 mmol/mol], impaired glucose tolerance, or impaired fasting glucose) should be tested yearly.[2] See Screening.
Clinical presentation
Symptomatic patients may present with: polyuria, polydipsia, polyphagia, or unintentional weight loss (usually when hyperglycemia is more severe, e.g., >300 mg/dL [>16.7 mmol/L]); fatigue; blurred vision; paresthesias; nocturia; skin infections (bacterial or candidal); urinary infections; or acanthosis nigricans. [Figure caption and citation for the preceding image starts]: Acanthosis nigricans involving the axillaFrom the collection of Melvin Chiu, MD; used with permission [Citation ends].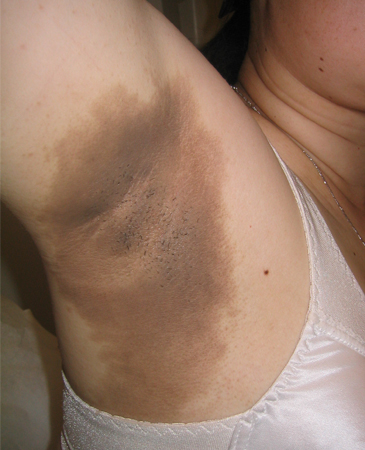
Because type 2 diabetes can often be present without diagnosis for many years, it is sometimes diagnosed when people present with microvascular complications, such as peripheral neuropathy, retinopathy, or nephropathy.
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) may also be the initial presentation of type 2 diabetes, particularly in ethnic and racial minorities, or if there is an underlying infection.[2][3] Patients are symptomatic of hyperglycemia (polyuria, polydipsia, weakness) and significant volume depletion (dry mucous membranes, poor skin turgor, tachycardia, hypotension, and, in severe cases, shock). This is a life-threatening emergency, which requires early diagnosis and management.[81] Compared with the acute presentation of DKA, people with HHS have an insidious onset (over days or weeks) of symptoms and are usually older than 60 years.[3] Certain medications, particularly antipsychotic agents, may precipitate HHS.[81] See Diabetic ketoacidosis and Hyperosmolar hyperglycemic state.
Diagnosis
Diagnosis can be made on the basis of any of the following:[2]
HbA1c ≥6.5% (≥48 mmol/mol); OR
Fasting plasma glucose (FPG) ≥126 mg/dL (≥7.0 mmol/L); OR
Plasma glucose ≥200 mg/dL (≥11.1 mmol/L) 2 hours after 75 g oral glucose; OR
Random plasma glucose of ≥200 mg/dL (≥11.1 mmol/L) in the presence of symptoms of hyperglycemia or hyperglycemic crisis.
In an asymptomatic patient, diagnosis requires two abnormal screening test results, measured either at the same time or at two different time points.[2] A single blood sample can therefore be used to establish a diabetes diagnosis if assays of both HbA1c and FPG meet the diagnostic criteria.[2] If using samples at two different time points, it is recommended that the second test, which may be either a repeat of the initial test or a different test, be performed promptly.[2] If an individual has discordant results from two different tests, then the test result that is above the diagnostic cut point should be repeated, with careful consideration of factors that may affect measured HbA1c or glucose levels. The diagnosis is made based on the confirmatory screening test. For example, if an individual meets the diabetes criterion of HbA1c (two results ≥6.5% [≥48 mmol/mol]) but not FPG (<126 mg/dL [<7.0 mmol/L]), that person should nevertheless be considered to have diabetes.[2]
In patients who have classic hyperglycemic symptoms (e.g., polyuria, polydipsia, and unexplained weight loss), measurement of a single random plasma glucose is sufficient to diagnose diabetes.[2] Healthcare professionals may also want to know the HbA1c to determine the chronicity of hyperglycemia.[2]
Some variability in HbA1c results is possible as a result of such factors as increased red blood cell turnover (e.g., sickle cell anemia), factors related to ancestry, or laboratory variation.[2] The ADA recommends that blood glucose criteria (and not HbA1c) should be used to diagnose diabetes in people with hemoglobinopathies including sickle cell disease, in women who are pregnant (second and third trimesters and the postpartum period), and those with glucose-6-phosphate dehydrogenase deficiency, HIV, hemodialysis, recent blood loss or transfusion, or on erythropoietin therapy. Marked discordance between measured HbA1c and plasma glucose levels should raise the possibility of HbA1c assay interference.[2] In older adults, an oral glucose tolerance test may identify impairments in glucose regulation more readily than other investigations, and this should be considered when screening this population.[25]
Differentiating type 1 and type 2 diabetes
Some individuals cannot be clearly classified as having type 1 or type 2 diabetes at the time of diagnosis due to disease heterogeneity.[2] However, at initial diagnosis of diabetes, it is important to determine if immediate treatment with insulin is required. Type 1 diabetes can occur at any age, but usually is diagnosed in younger patients (age <35 years) with lower body mass index (BMI; <25 kg/m²), unintentional weight loss, glucose >360 mg/dL (>20 mmol/L), and ketoacidosis.[2] Around one third of patients with newly diagnosed type 1 diabetes present with DKA.[82] However, DKA may also occur in type 2 diabetes, particularly in ethnic and racial minorities, or if there is an underlying infection.[2] Urine ketones should be checked if patients are symptomatic of hyperglycemia (polyuria, polydipsia, weakness) and volume depletion (dry mucous membranes, poor skin turgor, tachycardia, hypotension, and, in severe cases, shock) at diagnosis or throughout the course of disease. Although by definition HHS is characterized by negative ketone bodies, mild-to-moderate ketonemia may be present.[81]
C-peptide is produced in equal amounts to insulin and is the best measure of endogenous insulin secretion in patients with diabetes. There is no role for routine testing for C-peptide for diagnosis of diabetes, but its measurement may be useful in differentiating type 1 and type 2 diabetes.[83] The best evidenced C-peptide test is the glucagon stimulation test (GST), but nonfasting "random" blood C-peptide has been shown to correlate with fasting C-peptide and post-GST samples in subjects with well-defined type 1 or type 2 diabetes.[84] Development of absolute insulin deficiency is a key feature of type 1 diabetes, which results in low (<0.2 nmol/L) or undetectable levels of plasma C-peptide.[83] A GST or nonfasting "random" blood C-peptide level >1 nmol/L suggests type 2 diabetes.[83] C-peptide results must be interpreted in clinical context of disease duration, comorbidities, and family history.[84]
Although C-peptide can be helpful in evaluating the endogenous production of insulin, both type 1 and type 2 diabetes can be associated with insulinopenia, and endogenous insulin production can be detected in some individuals with type 1 diabetes for prolonged periods of time after diagnosis, especially in individuals diagnosed in adulthood. Testing for autoimmunity can therefore often be more helpful in identifying immune-mediated diabetes, the most prevalent form of type 1 diabetes. Autoantibodies to glutamic acid decarboxylase 65 (GAD65), islet cell antibodies (ICA), insulin antibodies, antibodies to tyrosine phosphatase-related islet antigen-2 (IA-2 and IA-2beta), and zinc-transporter-8 (ZnT8) antibodies can help to identify individuals with immune-mediated diabetes, although these antibodies fade with time after the onset of illness.[2]
Evaluation for macrovascular/microvascular complications
Assess the patient’s blood pressure, smoking status, and fasting lipid levels.
Take baseline urine albumin/creatinine ratio and serum creatinine with estimated glomerular filtration rate readings because signs of chronic kidney disease may be present at diagnosis.[2] A 2021 task force convened by the National Kidney Foundation and the American Society of Nephrology recommended the adoption of the new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Creatinine Equation (2021) that estimates kidney function without a race variable.[85] [ Glomerular Filtration Rate Estimation (eGFR) by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation with Creatinine, without Race (2021) Opens in new window ] This replaces the original CKD-EPI equation, which was previously widely used to estimate kidney function with an adjustment for black race.[85]
Check liver function tests. The ADA recommends screening all patients with type 2 diabetes or prediabetes for clinically significant fibrosis (defined as moderate fibrosis to cirrhosis) secondary to metabolic dysfunction-associated steatotic liver disease (also known as nonalcoholic fatty liver disease) using a calculated fibrosis-4 (FIB-4) index (derived from age, alanine aminotransferase, aspartate aminotransferase, and platelet count).[2] [ Cirrhosis probability in hepatitis C (FIB-4) Opens in new window ] Patients with an indeterminate or high FIB-4 index should have additional risk stratification by liver stiffness measurement with transient elastography, or by measurement of enhanced liver fibrosis, a blood biomarker.[2]
Ensure patients have an initial dilated and comprehensive eye exam by an eye specialist (e.g., ophthalmologist or optometrist). Patients with type 2 diabetes may have been undiagnosed for many years, and some manifestation of diabetic retinopathy is present in about 30% of patients.[2]
Examine the feet on diagnosis. This should include inspection of the skin, assessment of foot deformities, neurologic assessment (10-g monofilament testing with at least one other assessment: pinprick, temperature, vibration), and vascular assessment, including pulses in the legs and feet. Referral for ankle-brachial index (ABI) if pedal pulses are diminished.[2][77] Due to the potential for calcification of the arteries from atherosclerotic peripheral vascular disease (which falsely elevates the ABI), toe pressure testing is often done as an adjunct to ABI testing.[86] A normal ABI value is 1.0; a normal toe pressure value is over 0.7. Values below these levels are considered abnormal and are evidence of macrovascular arterial disease.
The ADA has developed a table summarizing components of the comprehensive medical evaluation for people diagnosed with diabetes, including recommended components to take place at initial, follow-up, and annual visits. American Diabetes Association: components of the comprehensive diabetes medical evaluation at initial, follow-up, and annual visits Opens in new window A comprehensive list is given, but the ADA specifies that in clinical practice, healthcare professionals may need to prioritize the components of the medical evaluation given the available resources and time.
For more detail on ongoing monitoring, see Monitoring.
Use of this content is subject to our disclaimer