The main goals are to cure the patient and to prevent further transmission of TB to others. The treating physician acts in a public health role as well and thus is responsible for ensuring that the patient successfully adheres to and completes treatment.
Treatment is initiated when TB is confirmed or strongly suspected and consists of an initial intensive phase and a subsequent continuation phase.
While infectious, patients should remain isolated (at home or in an appropriate room in the hospital). The treating physician should discuss the case with the local public health department to learn specific local requirements and to initiate a contact investigation in a timely fashion. Refer to guidelines for latest recommendations.
Latent TB infection
People who have had significant exposure in the previous 1-2 years should be evaluated for active TB disease and latent TB infection (LTBI; also sometimes referred to as TB infection). A repeat test for LTBI (TB skin test or interferon-gamma release assay) is recommended 8-10 weeks after the last exposure, if the initial evaluation was performed prior to this and the initial test result was negative. The decision whether to treat depends on the duration, proximity, and environment of exposure, as well as the immune status of the exposed contacts.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
For patients with LTBI that is presumed to be susceptible to isoniazid or rifampin, US guidelines' preferred regimens are rifamycin-based: 3 months of once-weekly isoniazid plus rifapentine, 4 months of daily rifampin, or 3 months of daily isoniazid plus rifampin.[44]Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021 Dec 1;148(6):e2021054663.
https://www.doi.org/10.1542/peds.2021-054663
http://www.ncbi.nlm.nih.gov/pubmed/34851422?tool=bestpractice.com
[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
[65]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Children with and Exposed to HIV. Guidelines for the prevention and treatment of opportunistic infections in children with and exposed to HIV: mycobacterium tuberculosis. 2023 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-pediatric-opportunistic-infections/mycobacterium-tuberculosis
[71]Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020 Feb 14;69(1):1-11.
https://www.cdc.gov/mmwr/volumes/69/rr/rr6901a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/32053584?tool=bestpractice.com
Three months of weekly isoniazid plus rifapentine given as directly observed therapy (DOT) or self-administered therapy (SAT) is recommended for adults and children ages >2 years, including those who have HIV infection where antiretroviral therapy (ART) drug interactions are acceptable.[44]Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021 Dec 1;148(6):e2021054663.
https://www.doi.org/10.1542/peds.2021-054663
http://www.ncbi.nlm.nih.gov/pubmed/34851422?tool=bestpractice.com
[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
[65]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Children with and Exposed to HIV. Guidelines for the prevention and treatment of opportunistic infections in children with and exposed to HIV: mycobacterium tuberculosis. 2023 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-pediatric-opportunistic-infections/mycobacterium-tuberculosis
[71]Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020 Feb 14;69(1):1-11.
https://www.cdc.gov/mmwr/volumes/69/rr/rr6901a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/32053584?tool=bestpractice.com
[72]Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):723-6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6023184
http://www.ncbi.nlm.nih.gov/pubmed/29953429?tool=bestpractice.com
[73]Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011 Dec 8;365(23):2155-66.
http://www.ncbi.nlm.nih.gov/pubmed/22150035?tool=bestpractice.com
[74]Belknap R, Holland D, Feng PJ, et al; TB Trials Consortium iAdhere Study Team. Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection: a randomized trial. Ann Intern Med. 2017 Nov 21;167(10):689-97.
http://www.ncbi.nlm.nih.gov/pubmed/29114781?tool=bestpractice.com
[75]Villarino ME, Scott NA, Weis SE; International Maternal Pediatric and Adolescents AIDS Clinical Trials Group; Tuberculosis Trials Consortium. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015 Mar;169(3):247-55.
http://www.ncbi.nlm.nih.gov/pubmed/25580725?tool=bestpractice.com
Safety of this regimen during pregnancy is not known, and is not recommended in pregnant women or those expecting to become pregnant during treatment. Four months of daily rifampin is recommended for adults and children of all ages and those with HIV infection where drug interactions allow; however, the US guidelines note that there is no evidence available for effectiveness of 4 months of daily rifampin in patients with HIV infection and it may be considered only as an alternative option to the other regimens in patients with HIV.[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
[71]Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020 Feb 14;69(1):1-11.
https://www.cdc.gov/mmwr/volumes/69/rr/rr6901a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/32053584?tool=bestpractice.com
Four months of daily rifampin was found to be noninferior in preventing TB, compared with 9 months of isoniazid in two randomized controlled trials, and was superior in safety and treatment completion.[76]Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018 Aug 2;379(5):440-53.
https://www.nejm.org/doi/full/10.1056/NEJMoa1714283
http://www.ncbi.nlm.nih.gov/pubmed/30067931?tool=bestpractice.com
[77]Diallo T, Adjobimey M, Ruslami R, et al. Safety and side effects of rifampin versus isoniazid in children. N Engl J Med. 2018 Aug 2;379(5):454-63.
https://www.nejm.org/doi/10.1056/NEJMoa1714284
http://www.ncbi.nlm.nih.gov/pubmed/30067928?tool=bestpractice.com
Three months of daily isoniazid plus rifampin is an option for adults and children of all ages and those with HIV infection where drug interactions allow.[71]Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020 Feb 14;69(1):1-11.
https://www.cdc.gov/mmwr/volumes/69/rr/rr6901a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/32053584?tool=bestpractice.com
Note that rifampin and rifapentine are not interchangeable between these regimens.
Regimens of daily or intermittent isoniazid for 6 or 9 months are alternative options for adults and children of all ages, including those with HIV infection. Intermittent regimens (i.e., twice weekly) should be given via directly observed therapy.[44]Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021 Dec 1;148(6):e2021054663.
https://www.doi.org/10.1542/peds.2021-054663
http://www.ncbi.nlm.nih.gov/pubmed/34851422?tool=bestpractice.com
[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
[71]Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020 Feb 14;69(1):1-11.
https://www.cdc.gov/mmwr/volumes/69/rr/rr6901a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/32053584?tool=bestpractice.com
The shorter course rifamycin-based regimens are preferred to isoniazid monotherapy, as they are more likely to be completed (risks of drug-induced hepatitis and treatment discontinuation are low). Isoniazid may be preferred when there are difficult to manage drug interactions between a patient’s other medications and the rifamycins.
Peripheral neuropathy is a common adverse effect of isoniazid due to pyridoxine antagonism. Pyridoxine supplementation should therefore be considered for prevention of peripheral neuropathy in patients with latent infection taking isoniazid, particularly for those in whom neuropathy is common (e.g., diabetes, uremia, alcoholism, malnutrition, HIV infection), for pregnant women, or for patients with seizure disorders.[21]American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000 Apr;161(4 Pt 2):S221-47.
https://www.atsjournals.org/doi/full/10.1164/ajrccm.161.supplement_3.ats600#.UoSlinC9lBE
http://www.ncbi.nlm.nih.gov/pubmed/10764341?tool=bestpractice.com
For patients with LTBI presumed to be due to contact with an infectious patient with drug resistant TB, expert consult should be sought.[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
[65]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Children with and Exposed to HIV. Guidelines for the prevention and treatment of opportunistic infections in children with and exposed to HIV: mycobacterium tuberculosis. 2023 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-pediatric-opportunistic-infections/mycobacterium-tuberculosis
[70]World Health Organization. WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment. Feb 2020 [internet publication].
https://www.who.int/publications/i/item/9789240001503
[78]Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019 Nov 15;200(10):e93-142.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31729908
http://www.ncbi.nlm.nih.gov/pubmed/31729908?tool=bestpractice.com
For patients exposed to isoniazid-resistant TB, 4 months of daily rifampin may be an option.[65]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Children with and Exposed to HIV. Guidelines for the prevention and treatment of opportunistic infections in children with and exposed to HIV: mycobacterium tuberculosis. 2023 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-pediatric-opportunistic-infections/mycobacterium-tuberculosis
[70]World Health Organization. WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment. Feb 2020 [internet publication].
https://www.who.int/publications/i/item/9789240001503
US guidelines recommend that patients with multidrug-resistant (MDR) TB are treated with 6-12 months of a fluoroquinolone (e.g., levofloxacin or moxifloxacin) alone or in combination with a second agent based on susceptibility testing of the source isolate.[78]Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019 Nov 15;200(10):e93-142.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31729908
http://www.ncbi.nlm.nih.gov/pubmed/31729908?tool=bestpractice.com
World Health Organization (WHO) guidelines recommend that, in selected high-risk household contacts of patients with multidrug resistant TB, preventive treatment may be considered based on individualized risk assessment and a sound clinical justification.[70]World Health Organization. WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment. Feb 2020 [internet publication].
https://www.who.int/publications/i/item/9789240001503
Active TB: intensive phase therapy (drug resistance not suspected)
Depending on the regimen, treatment for drug-susceptible active pulmonary TB is given for a total duration of 4, 6, or 9 months.
The initial intensive phase treatment for the standard 6- and 9-month regimens includes the preferred drugs of isoniazid, rifampin, pyrazinamide, and ethambutol, and lasts 8 weeks (2 months). Ethambutol may be discontinued immediately if the Mycobacterium tuberculosis isolate is sensitive to isoniazid and rifampin.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Pyrazinamide is used during the initial phase only and allows the treatment course to be shortened from 9 to 6 months. It is not recommended for patients with acute gouty arthritis (but can be used in patients with past history of gout), or in pregnant women because of little information about the safety data. Children ages between 3 months and 16 years with nonsevere TB (defined as TB confined to one lobe with no cavities or isolated intrathoracic adenopathy, no signs of miliary TB, no complex pleural effusion, and no clinically significant airway obstruction) may receive a 4-month version of this regimen (with daily administration of isoniazid, rifampin, and pyrazinamide, with or without ethambutol, for 2 months in the intensive phase).[79]Committee on Infectious Diseases, American Academy of Pediatrics. Tuberculosis. In: Kimberlin DW, Banerjee R, Barnett ED, ed(s). Red book: 2024-2027 report of the Committee on Infectious Diseases. 33rd ed. Itasca, IL: American Academy of Pediatrics; 2024.
Another 4-month regimen is available for those ages 12 years and over (with severe or nonsevere pulmonary TB). The intensive phase of this regimen includes daily administration of isoniazid, rifapentine, moxifloxacin, and pyrazinamide and also lasts 8 weeks.[80]Carr W, Kurbatova E, Starks A, et al. Interim guidance: 4-month rifapentine-moxifloxacin regimen for the treatment of drug-susceptible pulmonary tuberculosis - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022 Feb 25;71(8):285-9.
https://www.doi.org/10.15585/mmwr.mm7108a1
http://www.ncbi.nlm.nih.gov/pubmed/35202353?tool=bestpractice.com
[81]Dorman SE, Nahid P, Kurbatova EV, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med. 2021 May 6;384(18):1705-18.
https://www.nejm.org/doi/10.1056/NEJMoa2033400?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33951360?tool=bestpractice.com
Guidelines for the prevention and treatment of opportunistic infections in those with HIV recommend this regimen as an alternative option only for patients receiving an efavirenz-based ART regimen.[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
This regimen is not currently recommended for young children, persons who are pregnant, and patients with HIV infection and CD4 count of <100 cells/microliter or who are taking non-efavirenz-based ART.[80]Carr W, Kurbatova E, Starks A, et al. Interim guidance: 4-month rifapentine-moxifloxacin regimen for the treatment of drug-susceptible pulmonary tuberculosis - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022 Feb 25;71(8):285-9.
https://www.doi.org/10.15585/mmwr.mm7108a1
http://www.ncbi.nlm.nih.gov/pubmed/35202353?tool=bestpractice.com
Pyridoxine should be administered with isoniazid to help prevent isoniazid-associated neuropathy, and is recommended in all cases of active TB.
It is unlikely that adjunctive corticosteroid treatment provides major benefits for people with pulmonary TB, and thus its routine use is not recommended.[82]Critchley JA, Orton LC, Pearson F. Adjunctive steroid therapy for managing pulmonary tuberculosis. Cochrane Database Syst Rev. 2014 Nov 12;2014(11):CD011370.
https://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011370/full
http://www.ncbi.nlm.nih.gov/pubmed/25387839?tool=bestpractice.com
[  ]
What are the benefits and harms of steroids as adjuncts to standard treatment for pulmonary tuberculosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1170/fullShow me the answer
]
What are the benefits and harms of steroids as adjuncts to standard treatment for pulmonary tuberculosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1170/fullShow me the answer
Active TB: continuation phase therapy (drug resistance not suspected)
Patients completing the initial intensive regimen of isoniazid, rifampin, pyrazinamide, and ethambutol continue to receive isoniazid and rifampin in the continuation phase for 18 weeks (a total of 6-month [26 weeks] treatment) or 31 weeks (total of 9-month [39 weeks] treatment). Patients who should receive a total of 9-month treatment include:
Those who did not receive pyrazinamide during intensive phase
Those who have isoniazid- and rifampin-susceptible, but pyrazinamide-resistant TB
Those who have cavitary disease on chest x-ray and have positive sputum culture at 2 months into treatment.[Figure caption and citation for the preceding image starts]: Pulmonary TB with cavitationFrom the personal collection of David Horne and Masahiro Narita; used with permission [Citation ends].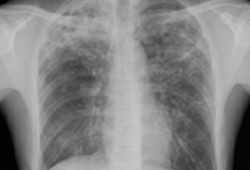
Extension of the continuation phase (to 9 months total) may also be considered in patients with either cavitary disease on chest x-ray or positive sputum culture at 8 weeks into treatment and who also: are HIV-positive; are underweight; have extensive disease on chest x-ray; have diabetes; or are active smokers.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Patients are considered infectious until:
Three consecutive sputum acid-fast bacilli smears are negative
They have been on standard therapy for at least 2 weeks
They show clinical improvement on TB therapy.
Children with nonsevere TB receiving the 4-month version of the isoniazid, rifampin, pyrazinamide, and ethambutol regimen should receive isoniazid and rifampin for 2 months in the continuation phase.[79]Committee on Infectious Diseases, American Academy of Pediatrics. Tuberculosis. In: Kimberlin DW, Banerjee R, Barnett ED, ed(s). Red book: 2024-2027 report of the Committee on Infectious Diseases. 33rd ed. Itasca, IL: American Academy of Pediatrics; 2024.
In the continuation phase of the isoniazid, rifapentine, moxifloxacin, pyrazinamide 4-month regimen, pyrazinamide is discontinued and isoniazid, rifapentine, and moxifloxacin are given for 9 weeks (total of 17 weeks [4 months]).[81]Dorman SE, Nahid P, Kurbatova EV, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med. 2021 May 6;384(18):1705-18.
https://www.nejm.org/doi/10.1056/NEJMoa2033400?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/33951360?tool=bestpractice.com
The recommended treatment duration is independent of any cavitation on baseline chest radiograph and there are no explicit recommendations on conditions under which treatment should be continued beyond 17 weeks in total. Patients with M tuberculosis positive cultures beyond 2 months of therapy, with or without ongoing symptoms, should be evaluated for causes of delayed response including drug susceptibility testing.[80]Carr W, Kurbatova E, Starks A, et al. Interim guidance: 4-month rifapentine-moxifloxacin regimen for the treatment of drug-susceptible pulmonary tuberculosis - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022 Feb 25;71(8):285-9.
https://www.doi.org/10.15585/mmwr.mm7108a1
http://www.ncbi.nlm.nih.gov/pubmed/35202353?tool=bestpractice.com
Mode of administration: active TB
To reduce nonadherence rates, therapy can be given by a healthcare professional in conjunction with a local public health authority as DOT. DOT can be given 5 days per week or 3 times weekly, depending on the regimen and phase of treatment. Although one systematic review has concluded that DOT does not provide a solution to poor adherence in TB treatment, the US and WHO guidelines recommend its use for all patients, especially in certain populations, such as drug-resistant disease; HIV coinfection; substance abuse; psychiatric sickness; children and adolescents; and others who, in the physician's opinion, might not adhere to SAT.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
[83]Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2015 May 29;2015(5):CD003343.
https://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003343.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/26022367?tool=bestpractice.com
[84]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment. May 2022 [internet publication].
https://www.who.int/publications/i/item/9789240048126
[85]Jasmer RM, Seaman CB, Gonzalez L, et al. Tuberculosis treatment outcomes: directly observed therapy compared with self administered therapy. Am J Respir Crit Care Med. 2004 Sep 1;170(5):561-6.
https://www.atsjournals.org/doi/full/10.1164/rccm.200401-095OC#.UoSkN3C9lBE
http://www.ncbi.nlm.nih.gov/pubmed/15184210?tool=bestpractice.com
[  ]
How does directly observed therapy affect outcomes in people being treated for tuberculosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1530/fullShow me the answer[Evidence B]3f1c5471-8490-4e07-af60-37476a641a96ccaBHow does directly observed therapy affect outcomes in people being treated for tuberculosis? Video DOT (vDOT) is the use of video calls to view patients ingesting their medications remotely. The Centers for Disease Control and Prevention (CDC) recommends the use of vDOT as equivalent to in-person DOT for patients undergoing TB treatment.[86]Mangan JM, Woodruff RS, Winston CA, et al. Recommendations for Use of Video Directly Observed Therapy During Tuberculosis Treatment - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023 Mar 24;72(12):313-316.
https://www.doi.org/10.15585/mmwr.mm7212a4
http://www.ncbi.nlm.nih.gov/pubmed/36952279?tool=bestpractice.com
In the WHO guidelines, the term "directly observed therapy (DOT)" has been replaced with "treatment support," which refers to any person (not necessarily a healthcare worker) observing the patient taking medication in real time, including via video.[87]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: tuberculosis care and support. Jul 2022 [internet publication].
https://www.who.int/publications/i/item/9789240047716
Intermittent therapy should be supervised. Nonsupervised therapy (SAT) should be daily, 7 days a week. Other regimens may be used; consult guidelines for details.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
]
How does directly observed therapy affect outcomes in people being treated for tuberculosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1530/fullShow me the answer[Evidence B]3f1c5471-8490-4e07-af60-37476a641a96ccaBHow does directly observed therapy affect outcomes in people being treated for tuberculosis? Video DOT (vDOT) is the use of video calls to view patients ingesting their medications remotely. The Centers for Disease Control and Prevention (CDC) recommends the use of vDOT as equivalent to in-person DOT for patients undergoing TB treatment.[86]Mangan JM, Woodruff RS, Winston CA, et al. Recommendations for Use of Video Directly Observed Therapy During Tuberculosis Treatment - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023 Mar 24;72(12):313-316.
https://www.doi.org/10.15585/mmwr.mm7212a4
http://www.ncbi.nlm.nih.gov/pubmed/36952279?tool=bestpractice.com
In the WHO guidelines, the term "directly observed therapy (DOT)" has been replaced with "treatment support," which refers to any person (not necessarily a healthcare worker) observing the patient taking medication in real time, including via video.[87]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: tuberculosis care and support. Jul 2022 [internet publication].
https://www.who.int/publications/i/item/9789240047716
Intermittent therapy should be supervised. Nonsupervised therapy (SAT) should be daily, 7 days a week. Other regimens may be used; consult guidelines for details.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Interruptions in treatment: active TB
Interruptions in therapy are common in the treatment of TB. The decision is then whether to restart a complete course of treatment or simply to continue. As a general guide, the earlier in the course of treatment and the greater the length of the lapse, the more likely the need to return to the beginning of the intensive phase of treatment.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Advice on how to manage interruptions to treatment with the 6- or 9-month regimens can be found in the American Thoracic Society/US CDC/Infectious Diseases Society of America guidelines, and are summarized below.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Treatment interrupted during the intensive phase:
Treatment interrupted during the continuation phase:
Received ≥80% of doses and sputum was acid-fast bacilli smear negative on initial testing - then no further therapy may be required
Received ≥80% of doses and sputum was acid-fast bacilli smear positive on initial testing - continue therapy and complete all doses
Received <80% of doses and accumulative lapse is <3 months in duration - continue therapy until all doses are completed, unless the consecutive lapse is >2 months. If treatment cannot be completed within the recommended time frame for the regimen, restart therapy from the beginning (i.e., from the intensive phase)
Received <80% of doses and lapse is ≥3 months in duration - restart therapy from the beginning (i.e., from the intensive phase).
Treatment with the 4-month regimen is considered complete when a total of 119 doses are taken. The 56 intensive phase doses should be administered within 70 days from treatment initiation, and 63 continuation phase doses should be administered within 84 days from intensive phase completion. Based on these requirements, the regimen should be completed within 5 months. Otherwise, the patient should be considered to have interrupted therapy and be managed as described above.
Patients with HIV co-infection
Treatment of HIV-positive patients is similar to that of non-HIV-positive patients. Therapy may be DOT or SAT, although DOT is highly recommended for HIV-positive patients.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Daily dosing is recommended throughout treatment for patients with HIV.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
[65]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Children with and Exposed to HIV. Guidelines for the prevention and treatment of opportunistic infections in children with and exposed to HIV: mycobacterium tuberculosis. 2023 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-pediatric-opportunistic-infections/mycobacterium-tuberculosis
[88]Gopalan N, Santhanakrishnan RK, Palaniappan AN, et al. Daily vs intermittent antituberculosis therapy for pulmonary tuberculosis in patients with HIV: A randomized clinical trial. JAMA Intern Med. 2018 Apr 1;178(4):485-93.
http://www.ncbi.nlm.nih.gov/pubmed/29507938?tool=bestpractice.com
Patients with HIV can be maintained on standard therapy, but rifabutin may be considered in place of rifampin, depending on the ART regimen the patient is on.
Rifabutin has less effect on the serum concentrations of protease inhibitors than rifampin. Patients on protease-inhibitor based ART regimens should receive rifabutin instead of rifampin in their TB-treatment regimen. Specialist consultation is recommended when considering use of rifabutin while the patient is on a protease-inhibitor.
The initiation of ART as soon as possible after starting treatment for TB is consistent with WHO guidelines.[89]World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. Nov 2009 [internet publication].
http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf
[90]Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010 Jul 15;363(3):257-65.
http://www.nejm.org/doi/full/10.1056/NEJMoa0910370#t=article
http://www.ncbi.nlm.nih.gov/pubmed/20647201?tool=bestpractice.com
[91]Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010 Feb 25;362(8):697-706.
http://www.nejm.org/doi/full/10.1056/NEJMoa0905848#t=article
http://www.ncbi.nlm.nih.gov/pubmed/20181971?tool=bestpractice.com
Survival is improved in co-infected individuals with CD4+ counts of <50 cells/microliter if ART is initiated within 2 weeks of starting TB treatment.[92]Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011 Oct 20;365(16):1492-501.
https://www.nejm.org/doi/full/10.1056/NEJMoa1014181#t=article
http://www.ncbi.nlm.nih.gov/pubmed/22010915?tool=bestpractice.com
[93]Havlir DV, Kendall MA, Ive P, et al; AIDS Clinical Trials Group Study A5221. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011 Oct 20;365(16):1482-91.
http://www.nejm.org/doi/full/10.1056/NEJMoa1013607#t=article
http://www.ncbi.nlm.nih.gov/pubmed/22010914?tool=bestpractice.com
[94]Uthman OA, Okwundu C, Gbenga K, et al. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: a systematic review and meta-analysis. Ann Intern Med. 2015 Jul 7;163(1):32-9.
http://www.ncbi.nlm.nih.gov/pubmed/26148280?tool=bestpractice.com
If a patient co-infected with HIV and TB is not started on ART, then consideration should be given to extending TB treatment to 9 months (i.e., additional 3 months of continuation phase).[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Extension of the continuation phase should also be considered in patients with either cavitary disease on chest x-ray or positive sputum culture at 2 months into treatment and HIV co-infection.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Management of patients with additional comorbidities is complex, and will require specialist advice.
Impaired renal function
Patients who have reduced renal function but have a creatinine clearance >30 mL/minute should receive medications in the standard doses. However, monitoring serum drug levels should be considered.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
In patients with a creatinine clearance of <30 mL/minute, ethambutol and pyrazinamide are given three times a week (dose given after hemodialysis if the patient is on dialysis; expert consultation recommended).[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Drug-induced hepatitis or pre-existing liver disease
Tests for hepatitis A, B, and C should be ordered if the patient has no pre-existing liver disease but has abnormal liver function test results. Liver function (aminotransferases, bilirubin, alkaline phosphatase) should be checked at baseline. Monthly liver function tests (LFTs) should be obtained in patients with abnormal baseline liver function, underlying liver disease, HIV coinfection, and other risk factors for hepatitis. Patients should be asked about other hepatotoxins, including alcohol use.
Isoniazid, rifampin, and pyrazinamide can all cause or exacerbate liver disease. The decision about whether it is safe to continue one, two, or all three of these drugs depends on severity of liver disease. An asymptomatic increase in alanine aminotransferase (ALT) occurs in 20% of patients treated with regimens containing these drugs. If this is less than five times upper limit of normal (ULN) with no symptoms, or under three times ULN with symptoms, first-line regimens can be continued, but LFTs and symptoms should be monitored.
Where ALT increase is more than five times ULN, or more than three times ULN with symptoms, hepatotoxic drugs should be stopped and treatment initiated with at least three drugs without hepatotoxic effects (e.g., ethambutol, fluoroquinolone, and linezolid) especially if the burden of TB is more than minimal. It may be possible to continue with one or two of the more effective drugs, isoniazid and/or rifampin, with careful monitoring of liver function, especially when ALT becomes less than two times ULN. These can be serially reintroduced, one by one, waiting 4-7 days before adding next drug. Before introducing each new drug, LFTs should be checked. If an increase in ALT occurs, the most recently introduced drug is likely responsible for the hepatitis.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
[95]Manosuthi W, Kiertiburanakul S, Phoorisri T, et al. Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J Infect. 2006 Dec;53(6):357-63.
http://www.ncbi.nlm.nih.gov/pubmed/16487593?tool=bestpractice.com
Although rifabutin may cause hepatic injury (mainly a cholestatic pattern like rifampin), it has been found to be less hepatotoxic compared with rifampin and may be substituted for rifampin in order to achieve short-course TB regimen. For example, if liver injury recurs with the reintroduction of rifampin, rifabutin may be considered as a replacement.
Management of patients with additional comorbidities is complex, and will require specialist advice.
Pregnancy and breast-feeding
Pregnant women who are suspected of having active TB should be treated as there is a risk of TB transmission to the fetus. Initial treatment is normally with isoniazid, rifampin, and ethambutol. Pyrazinamide is probably safe and can be considered in addition to triple therapy, especially for patients with HIV infection or high bacillary burden. The minimum total duration of treatment is 9 months for women who did not receive pyrazinamide as part of the initial regimen.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
The 4-month rifapentine-moxifloxacin regimen is not recommended for pregnant patients. Those who become pregnant while on the 4-month treatment regimen may need to stop and switch to an alternative regimen; clinical consultation is recommended.[80]Carr W, Kurbatova E, Starks A, et al. Interim guidance: 4-month rifapentine-moxifloxacin regimen for the treatment of drug-susceptible pulmonary tuberculosis - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022 Feb 25;71(8):285-9.
https://www.doi.org/10.15585/mmwr.mm7108a1
http://www.ncbi.nlm.nih.gov/pubmed/35202353?tool=bestpractice.com
Women taking antituberculosis treatment can breast-feed because only low levels of drugs are passed into the milk. However, levels are not high enough to provide effective treatment for the baby.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Management of patients with additional comorbidities is complex, and will require specialist advice.
Isoniazid-resistant TB
Isoniazid-resistant TB is defined as resistance to isoniazid and susceptibility to rifampin that has been confirmed in vitro. In patients with confirmed rifampin-susceptible and isoniazid-resistant TB, US and WHO guidelines recommend treatment with rifampin, ethambutol, pyrazinamide, and a later generation fluoroquinolone (i.e., levofloxacin or moxifloxacin; WHO recommends levofloxacin) for a duration of 6 months.[78]Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019 Nov 15;200(10):e93-142.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31729908
http://www.ncbi.nlm.nih.gov/pubmed/31729908?tool=bestpractice.com
[96]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment, 2022 update. Dec 2022 [internet publication].
https://www.who.int/publications/i/item/9789240063129
If a fluoroquinolone cannot be used (because of toxicity or resistance) WHO recommends that the patient is treated with rifampin, ethambutol, and pyrazinamide for 6 months.[96]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment, 2022 update. Dec 2022 [internet publication].
https://www.who.int/publications/i/item/9789240063129
As pyrazinamide may be a common cause of adverse effects, the US guidelines suggest that when pyrazinamide toxicity is experienced or anticipated, or when the bacillary burden is low (e.g., noncavitary disease), pyrazinamide may be discontinued after 2 months of therapy.
WHO guidelines state that resistance to rifampin must be excluded before starting the regimen, and preferably, resistance to fluoroquinolones and pyrazinamide should also be excluded. If isoniazid resistance is identified after a patient has started a regimen for drug-susceptible TB, rapid molecular testing for rifampin resistance should be done, and if rifampin resistance is excluded, the patient should begin a full 6-month course of rifampin, ethambutol, pyrazinamide, and a later-generation fluoroquinolone (i.e., levofloxacin or moxifloxacin). If rifampin resistance is detected, the patient should begin an appropriate treatment regimen for MDR TB.[96]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment, 2022 update. Dec 2022 [internet publication].
https://www.who.int/publications/i/item/9789240063129
In contrast to regimens for drug-susceptible and MDR TB, the recommended treatment regimen for isoniazid-resistant TB does not have initial intensive and continuation phases.
Multidrug-resistant TB
MDR TB is defined as resistance to isoniazid and rifampin, with or without resistance to other first-line drugs. Extensively drug-resistant (XDR) TB is defined as resistance to at least isoniazid and rifampin, as well as any fluoroquinolone and either bedaquiline or linezolid (or both).[97]World Health Organization. WHO announces updated definitions of extensively drug-resistant tuberculosis. Jan 2021 [internet publication].
https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis
Pre-XDR-TB is resistance to isoniazid, rifampin, and any fluoroquinolone.
Drug resistance may be suspected on the basis of historical or epidemiologic information, but should be considered in every patient.[78]Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019 Nov 15;200(10):e93-142.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31729908
http://www.ncbi.nlm.nih.gov/pubmed/31729908?tool=bestpractice.com
Management requires expert consultation and the final regimen should be based on the results of drug susceptibility testing.
US guidelines now recommend that at least five drugs are used in the intensive phase of treatment, and four drugs in the continuation phase.[78]Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019 Nov 15;200(10):e93-142.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31729908
http://www.ncbi.nlm.nih.gov/pubmed/31729908?tool=bestpractice.com
The duration of treatment will vary; however, the intensive phase is 5-7 months after culture conversion, and treatment then continues for a total of 15-21 months (after culture conversion) or 15-24 months for XDR TB.[78]Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019 Nov 15;200(10):e93-142.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31729908
http://www.ncbi.nlm.nih.gov/pubmed/31729908?tool=bestpractice.com
The regimen should include the following oral agents:
If the regimen cannot be assembled with five effective oral drugs, one of the following injectable agents may be used (depending on susceptibility testing):
Agents that may be used in place of the injectables include:
Pyrazinamide
Ethambutol
Delamanid (note: approved for the treatment of MDR-TB by the European Medicines Agency in 2013 but has not yet received FDA approval; the US guideline writing committee agrees with the WHO consolidated guidelines on drug-resistant tuberculosis treatment that delamanid may be included in the treatment of patients with MDR/rifampin-resistant (RR)-TB ages ≥3 years on longer regimens).[96]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment, 2022 update. Dec 2022 [internet publication].
https://www.who.int/publications/i/item/9789240063129
If more effective options are not available to assemble a regimen of five drugs, then the following options may be considered:
Ethionamide or prothionamide
A carbapenem (i.e., imipenem/cilastatin or meropenem) plus clavulanate (note: clavulanate is only available as a co-formulation with amoxicillin; therefore, amoxicillin/clavulanate must be given with the carbapenem when using this regimen)
Aminosalicylic acid
High-dose isoniazid.
Pretomanid has been approved for the treatment of XDR, treatment-intolerant, or nonresponsive MDR pulmonary TB, when used in combination with bedaquiline and linezolid (the BPaL regimen).[98]Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020 Mar 5;382(10):893-902.
https://www.doi.org/10.1056/NEJMoa1901814
http://www.ncbi.nlm.nih.gov/pubmed/32130813?tool=bestpractice.com
The CDC now recommends that BPaL may be used in the treatment of adults with pulmonary XDR or pre-XDR (resistant to isoniazid, rifampin, and at least one fluoroquinolone or injectable medication [i.e., amikacin, kanamycin, capreomycin]) or treatment-intolerant/nonresponsive MDR TB when a safe and effective treatment regimen cannot otherwise be provided.[99]Centers for Disease Control and Prevention. Provisional CDC guidance for the use of pretomanid as part of a regimen [bedaquiline, pretomanid, and linezolid (BPaL)] to treat drug-resistant tuberculosis disease. Feb 2024 [internet publication].
https://www.cdc.gov/tb/hcp/treatment/bpal.html
Guidelines for the prevention and treatment of opportunistic infections in those with HIV also now recommend BPaLM, which is a regimen consisting of bedaquiline, pretomanid, linezolid, and moxifloxacin, as the preferred option for rifampin-resistant pulmonary TB in people with HIV.[64]National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: mycobacterium tuberculosis infection and disease. 2024 [internet publication].
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium
The WHO guideline on MDR TB includes recommendations for short (6 or 9 months) and longer (18 months or more) regimens for the treatment of people with drug-resistant TB.[96]World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment, 2022 update. Dec 2022 [internet publication].
https://www.who.int/publications/i/item/9789240063129
The short-course regimens are a major step forward for low- and middle-income settings where access to second-line drug susceptibility testing may not be available. In places with the ability to check second-line drug sensitivities (as is the case in the US), creation of an appropriate regimen would be based on drug susceptibilities. The short-course regimens may expose patients to drugs that are not indicated. The US guideline makes no recommendation for or against a standardized shorter-course regimen at this time.[78]Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019 Nov 15;200(10):e93-142.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31729908
http://www.ncbi.nlm.nih.gov/pubmed/31729908?tool=bestpractice.com
Management of patients with additional comorbidities is complex, and requires specialist advice.
Safety of fluoroquinolones
Systemic fluoroquinolone antibiotics are a key part of some TB treatment regimens, but it is important to note that they may cause serious, disabling, and potentially long-lasting or irreversible adverse events. This includes, but is not limited to: tendinopathy/tendon rupture; peripheral neuropathy; arthropathy/arthralgia; aortic aneurysm and dissection; heart valve regurgitation; dysglycemia; and central nervous system effects including seizures, depression, psychosis, and suicidal thoughts and behavior.[100]Rusu A, Munteanu AC, Arbănași EM, et al. Overview of side-effects of antibacterial fluoroquinolones: new drugs versus old drugs, a step forward in the safety profile? Pharmaceutics. 2023 Mar 1;15(3):804.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10056716
http://www.ncbi.nlm.nih.gov/pubmed/36986665?tool=bestpractice.com
Prescribing restrictions apply to the use of fluoroquinolones, and these restrictions may vary between countries. In general, fluoroquinolones should be restricted for use in serious, life-threatening bacterial infections only. Some regulatory agencies may also recommend that they must only be used in situations where other antibiotics, that are commonly recommended for the infection, are inappropriate (e.g., resistance, contraindications, treatment failure, unavailability).
Consult your local guidelines and drug formulary for more information on suitability, contraindications, and precautions.
Treatment failure and TB recurrence
WHO defines treatment failure as a positive sputum smear or culture at 5 months or later during TB treatment.[59]World Health Organization. Definitions and reporting framework for tuberculosis - 2013 revision (updated December 2014 and January 2020). Jan 2020 [internet publication].
https://apps.who.int/iris/handle/10665/79199
When either the sputum smear or culture remains positive beyond 2 to 3 months into TB treatment, adherence with TB medications must be verified. Emerging drug-resistant TB during treatment and gastrointestinal malabsorption of TB medications should also be evaluated.
Recurrence of TB occurs in a case previously considered to have been successfully treated for TB. Recurrent cases include relapses due to the same M tuberculosis strain as that responsible for the previous episode, as well as new episodes of TB due to re-exposure resulting in reinfection. In the US, recurrence is generally a result of recrudescence of the original organism (i.e., relapse), whereas in TB-endemic countries, it may be the result of exogenous reinfection. Most relapse events occur in the first 6 to 12 months following completion of treatment and occur in 2% to 5% of appropriately treated patients.[101]Tuberculosis Trials Consortium. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002 Aug 17;360(9332):528-34.
http://www.ncbi.nlm.nih.gov/pubmed/12241657?tool=bestpractice.com
If the patient initially had drug-susceptible isolates and treatment was directly observed, recurrence will likely be the result of the same susceptible organisms and prior therapy can be used. However, if the patient initially received SAT, there is a greater possibility of the development of a drug-resistant organism. In this situation, or if drug susceptibility has not previously been tested, an expanded MDR TB regimen with addition of at least 2 new drugs not previously used should be considered.
If exogenous reinfection is suspected, TB treatment should be based on the drug susceptibility profile of the index case, if known.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
 ]
]

 ]
[Evidence B] Video DOT (vDOT) is the use of video calls to view patients ingesting their medications remotely. The Centers for Disease Control and Prevention (CDC) recommends the use of vDOT as equivalent to in-person DOT for patients undergoing TB treatment.[86] In the WHO guidelines, the term "directly observed therapy (DOT)" has been replaced with "treatment support," which refers to any person (not necessarily a healthcare worker) observing the patient taking medication in real time, including via video.[87] Intermittent therapy should be supervised. Nonsupervised therapy (SAT) should be daily, 7 days a week. Other regimens may be used; consult guidelines for details.[9]
]
[Evidence B] Video DOT (vDOT) is the use of video calls to view patients ingesting their medications remotely. The Centers for Disease Control and Prevention (CDC) recommends the use of vDOT as equivalent to in-person DOT for patients undergoing TB treatment.[86] In the WHO guidelines, the term "directly observed therapy (DOT)" has been replaced with "treatment support," which refers to any person (not necessarily a healthcare worker) observing the patient taking medication in real time, including via video.[87] Intermittent therapy should be supervised. Nonsupervised therapy (SAT) should be daily, 7 days a week. Other regimens may be used; consult guidelines for details.[9]