The diagnosis may be obvious in some cases but is frequently difficult. A high level of suspicion is important in evaluating a patient with risk factors. Diagnosis confirmation requires culturing of Mycobacterium tuberculosis. Delays in diagnosis and initiation of therapy are associated with transmission of disease and increased mortality.[12]Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):e1-33.
https://academic.oup.com/cid/article/64/2/e1/2629583
http://www.ncbi.nlm.nih.gov/pubmed/27932390?tool=bestpractice.com
If suspicion for disease is high, the patient should be isolated (at home or in a negative-pressure room in a hospital) until 5 days to 2 weeks of therapy has been completed. Active TB, confirmed or highly suspected, is a reportable condition to the local health authorities.
Clinical history and risk factors
The possibility of TB should be considered in any person with risk factors for TB exposure, who has suggestive symptoms (e.g., fever, malaise, pleuritic chest pain, cough longer than 2-3 weeks, night sweats, and weight loss, hemoptysis, psychological symptoms, clubbing, erythema nodosum) or chest x-ray abnormalities. Although the presence of upper lobe infiltrates is characteristic of the disease, atypical chest x-ray presentation is common among children, and among people who are immunocompromised, have HIV infection, or have diabetes. [Figure caption and citation for the preceding image starts]: Pulmonary TB with cavitationFrom the personal collection of David Horne and Masahiro Narita; used with permission [Citation ends].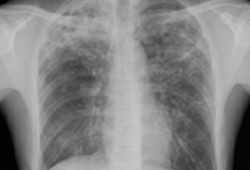
Diagnostic investigations
Investigations for active infection include chest x-ray, three sputum samples obtained for acid-fast bacilli (AFB), nucleic acid amplification testing (NAAT), complete blood count, and electrolytes (e.g., sodium). If the patient is unable to spontaneously produce sputum, it should be induced (with appropriate precautions to prevent transmission) or obtained via bronchoscopy or gastric aspirate.[12]Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):e1-33.
https://academic.oup.com/cid/article/64/2/e1/2629583
http://www.ncbi.nlm.nih.gov/pubmed/27932390?tool=bestpractice.com
Stained smears should be made from sputum specimens to identify AFB, as this is the first bacteriologic evidence of infection and gives an estimate of how infectious the patient is. If AFBs are seen on smear, therapy should be started and the patient maintained in isolation.
Sputum culture supports the diagnosis of TB, is more sensitive and specific than smear staining, facilitates identification of the mycobacterium species by nucleic acid hybridization or amplification, and evaluates drug sensitivity. Broth culture systems allow for rapid growth and detection in 1 to 3 weeks as opposed to 4 to 8 weeks by a solid medium.[12]Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):e1-33.
https://academic.oup.com/cid/article/64/2/e1/2629583
http://www.ncbi.nlm.nih.gov/pubmed/27932390?tool=bestpractice.com
NAAT should be performed on at least one respiratory specimen when a diagnosis of TB is being considered. NAAT may speed the diagnosis in smear-negative cases and may be helpful to differentiate nontuberculous mycobacteria when sputum is AFB smear positive but NAAT negative.[31]Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009 Jan 16;58(1):7-10.
https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5801a3.htm
http://www.ncbi.nlm.nih.gov/pubmed/19145221?tool=bestpractice.com
Genotyping might be considered useful in outbreaks of TB to identify recent transmission of TB, especially when contact had not been identified in the course of epidemiologic investigations. Several rapid NAATs are available and some are also able to detect genes encoding resistance to TB drugs.[32]World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 3rd ed. Mar 2024 [internet publication].
https://www.who.int/publications/i/item/9789240089488
[33]Zifodya JS, Kreniske JS, Schiller I, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021 Feb 22;(2):CD009593.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009593.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/33616229?tool=bestpractice.com
[34]Shapiro AE, Ross JM, Yao M, et al. Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms. Cochrane Database Syst Rev. 2021 Mar 23;(3):CD013694.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013694.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/33755189?tool=bestpractice.com
[35]Pillay S, Steingart KR, Davies GR, et al. Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin. Cochrane Database Syst Rev. 2022 May 18;(5):CD014841.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014841.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/35583175?tool=bestpractice.com
[36]Haraka F, Kakolwa M, Schumacher SG, et al. Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis. Cochrane Database Syst Rev. 2021 May 6;(5):CD012972.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012972.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/34097769?tool=bestpractice.com
[37]Kay AW, Ness T, Verkuijl SE, et al. Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children. Cochrane Database Syst Rev. 2022 Sep 6;(9):CD013359.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013359.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/36065889?tool=bestpractice.com
[38]World Health Organization. WHO consolidated guidelines on tuberculosis: module 5: management of tuberculosis in children and adolescents. Mar 2022 [internet publication].
https://www.who.int/publications/i/item/9789240046764
A positive NAAT is adequate for initiation of antituberculosis treatment. When the patient has previously been treated for TB, especially within the prior 2 years, a positive NAAT may represent a false-positive result.
CT of the chest, although not done routinely, may be of use to exclude other pathology: for example, cancer.
Lateral flow tests that detect lipoarabinomannan (LAM) antigen in urine have emerged as potential point-of-care tests. One Cochrane review found the lateral flow urine lipoarabinomannan (LF-LAM) assay to have a sensitivity of 42% in diagnosing TB in HIV-positive individuals with TB symptoms, and 35% in HIV-positive individuals not assessed for TB symptoms.[39]Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev. 2019 Oct 21;(10):CD011420.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011420.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/31633805?tool=bestpractice.com
[  ]
What is the accuracy of lateral flow urine lipoarabinomannan (LF‐LAM) for detecting tuberculosis (TB) in unselected HIV‐positive adults (symptomatic or asymptomatic)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2825/fullShow me the answer
[
]
What is the accuracy of lateral flow urine lipoarabinomannan (LF‐LAM) for detecting tuberculosis (TB) in unselected HIV‐positive adults (symptomatic or asymptomatic)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2825/fullShow me the answer
[  ]
What is the accuracy of lateral flow urine lipoarabinomannan (LF‐LAM) for detecting tuberculosis (TB) in symptomatic HIV‐positive adults?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2824/fullShow me the answer The World Health Organization (WHO) recommends that LF-LAM can be used to assist in the diagnosis of active TB in HIV-positive adults, adolescents, and children.[32]World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 3rd ed. Mar 2024 [internet publication].
https://www.who.int/publications/i/item/9789240089488
This approach is supported by another Cochrane review, which found reductions in mortality and an increase in treatment initiation with use of LF-LAM in inpatient and outpatient settings.[40]Nathavitharana RR, Lederer P, Chaplin M, et al. Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV. Cochrane Database Syst Rev. 2021 Aug 20;(8):CD014641.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014641/full
http://www.ncbi.nlm.nih.gov/pubmed/34416013?tool=bestpractice.com
Culture would still be required for drug susceptibility testing (DST).
]
What is the accuracy of lateral flow urine lipoarabinomannan (LF‐LAM) for detecting tuberculosis (TB) in symptomatic HIV‐positive adults?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2824/fullShow me the answer The World Health Organization (WHO) recommends that LF-LAM can be used to assist in the diagnosis of active TB in HIV-positive adults, adolescents, and children.[32]World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 3rd ed. Mar 2024 [internet publication].
https://www.who.int/publications/i/item/9789240089488
This approach is supported by another Cochrane review, which found reductions in mortality and an increase in treatment initiation with use of LF-LAM in inpatient and outpatient settings.[40]Nathavitharana RR, Lederer P, Chaplin M, et al. Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV. Cochrane Database Syst Rev. 2021 Aug 20;(8):CD014641.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014641/full
http://www.ncbi.nlm.nih.gov/pubmed/34416013?tool=bestpractice.com
Culture would still be required for drug susceptibility testing (DST).
While not routinely used in the US, use of stool samples is an alternative to respiratory specimens in the diagnosis of pulmonary TB (sputum is swallowed and M tuberculosis may pass through the gastrointestinal tract). One systematic review evaluating AFB-smear, culture, and NAAT (polymerase chain reaction [PCR]) testing of stool in pulmonary TB found a pooled sensitivity of one or more of the three tests was 79.1% (95% CI 61.5 to 92.5).[41]Laursen LL, Dahl VN, Wejse C. Stool testing for pulmonary TB diagnosis in adults. Int J Tuberc Lung Dis. 2022 Jun 1;26(6):516-23.
http://www.ncbi.nlm.nih.gov/pubmed/35650697?tool=bestpractice.com
The sensitivity of stool microscopy, PCR, and culture was 41.1% (95% CI 24.9 to 58.2), 89.7% (95% CI 81.4 to 95.9), and 38.0% (95% CI 26.2 to 50.6), respectively.[41]Laursen LL, Dahl VN, Wejse C. Stool testing for pulmonary TB diagnosis in adults. Int J Tuberc Lung Dis. 2022 Jun 1;26(6):516-23.
http://www.ncbi.nlm.nih.gov/pubmed/35650697?tool=bestpractice.com
It is recommended that all patients who have TB should be tested for HIV within 2 months of diagnosis.
Negative smear or cultures
Around 40% to 50% of cases are AFB smear negative and 15% to 20% have negative cultures. In patients where there is a strong clinical suspicion of TB, especially if the tuberculin skin test is positive (reaction of ≥5 mm induration), empiric TB therapy may be tried before laboratory confirmation of infection. Clinical and radiographic improvement with appropriate antituberculosis treatment supports the diagnosis. NAAT of sputum cultures may also be helpful in this situation. Bronchoscopy can be done to obtain bronchoalveolar lavage samples or transbronchial biopsy, and gastric aspirate can be used in patients unable to provide an adequate sputum sample, such as young children. In patients where suspicion for active TB is low and smears are negative for AFB, it is acceptable to wait for the results of AFB culture or repeat chest x-ray before starting treatment.[9]Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016 Oct;63(7):e147-95.
https://academic.oup.com/cid/article/63/7/e147/2196792
http://www.ncbi.nlm.nih.gov/pubmed/27516382?tool=bestpractice.com
Patients with smear-negative disease may be infectious, although the risk of transmission is lower than in smear-positive disease.[42]Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999 Feb 6;353(9151):444-9.
http://www.ncbi.nlm.nih.gov/pubmed/9989714?tool=bestpractice.com
If the suspicion of TB is high, consideration should be given to starting antituberculous medications prior to laboratory confirmation.
Susceptibility testing
In adequately resourced settings, culture-based DST is routinely performed on initial M tuberculosis isolates. A limitation of culture-based DST is that it can take >2 weeks to grow the isolate for testing. When there is a higher suspicion for drug resistance, then rapid molecular DST may be appropriate to guide treatment. Rapid molecular DST for rifampin with or without isoniazid using the respiratory specimens of persons who are either AFB smear positive or NAAT positive should be considered in patients who:
Have been treated for TB in the past, or
Were born in or have lived for at least 1 year in a foreign country with at least a moderate TB incidence (≥20 per 100,000) or a high primary multidrug-resistant (MDR) TB prevalence (≥2%), or
Are contacts of patients with MDR TB, or
Are HIV-positive.
When Xpert MTB/RIF or Xpert Ultra (rapid molecular tests endorsed by the WHO) are used as part of TB diagnosis, rifampin resistance will be automatically assessed.[12]Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):e1-33.
https://academic.oup.com/cid/article/64/2/e1/2629583
http://www.ncbi.nlm.nih.gov/pubmed/27932390?tool=bestpractice.com
[32]World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 3rd ed. Mar 2024 [internet publication].
https://www.who.int/publications/i/item/9789240089488
Cochrane reviews of Xpert MTB/RIF and Xpert Ultra found that they provide accurate results for rifampin‐resistant and MDR TB.[33]Zifodya JS, Kreniske JS, Schiller I, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021 Feb 22;(2):CD009593.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009593.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/33616229?tool=bestpractice.com
[34]Shapiro AE, Ross JM, Yao M, et al. Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms. Cochrane Database Syst Rev. 2021 Mar 23;(3):CD013694.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013694.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/33755189?tool=bestpractice.com
[37]Kay AW, Ness T, Verkuijl SE, et al. Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children. Cochrane Database Syst Rev. 2022 Sep 6;(9):CD013359.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013359.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/36065889?tool=bestpractice.com
Xpert MTB/XDR assesses resistance to isoniazid, fluoroquinolones, injectables (amikacin, kanamycin, capreomycin), and ethionamide. One Cochrane review found Xpert MTB/XDR is accurate for detecting isoniazid and fluoroquinolone resistance.[35]Pillay S, Steingart KR, Davies GR, et al. Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin. Cochrane Database Syst Rev. 2022 May 18;(5):CD014841.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014841.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/35583175?tool=bestpractice.com
Line probe assays (LPAs) are strip-based tests that can detect TB and determine drug resistance profiles. LPAs are recommended by WHO only for detecting resistance to anti-TB drugs.[32]World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 3rd ed. Mar 2024 [internet publication].
https://www.who.int/publications/i/item/9789240089488
WHO also now includes next-generation sequencing (NGS) in its recommendations for DST in confirmed pulmonary TB disease.[32]World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 3rd ed. Mar 2024 [internet publication].
https://www.who.int/publications/i/item/9789240089488
NGS is a new class of test that can be used for the detection of resistance to a broad list of drugs.
Tuberculin skin test and interferon gamma release assays
Investigations for latent infection in a person exposed to M tuberculosis but without signs of active TB are based on the tuberculin skin test (TST) or interferon gamma release assays (IGRAs). The TST and IGRA measure the response of T-cells to TB antigens. As false-negative results occur in 20% to 25% of patients with active pulmonary TB, these tests should not be used alone to exclude a diagnosis of active TB.[43]Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011 Nov 15;204 (suppl 4):S1120-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3192542
http://www.ncbi.nlm.nih.gov/pubmed/21996694?tool=bestpractice.com
The American Academy of Pediatrics advises that a negative result of either TST or IGRA should be considered especially unreliable in a child younger than 3 months.[44]Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021 Dec 1;148(6):e2021054663.
https://www.doi.org/10.1542/peds.2021-054663
http://www.ncbi.nlm.nih.gov/pubmed/34851422?tool=bestpractice.com
Interpretation of the TST depends on patient characteristics including immunocompetence and vaccination status. For patients with normal immunity and no additional risk factors, induration of ≥15 mm in diameter is taken to mean a positive result, but a smaller diameter is used as a cut-off in people with additional risk factors and in children.[12]Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):e1-33.
https://academic.oup.com/cid/article/64/2/e1/2629583
http://www.ncbi.nlm.nih.gov/pubmed/27932390?tool=bestpractice.com
[38]World Health Organization. WHO consolidated guidelines on tuberculosis: module 5: management of tuberculosis in children and adolescents. Mar 2022 [internet publication].
https://www.who.int/publications/i/item/9789240046764
[45]Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008 Aug 5;149(3):177-84.
http://www.ncbi.nlm.nih.gov/pubmed/18593687?tool=bestpractice.com
An IGRA may be used in place of a TST in all situations in which TSTs are used to diagnose latent infection, though TST may be preferred in children ages younger than 2 years.[44]Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021 Dec 1;148(6):e2021054663.
https://www.doi.org/10.1542/peds.2021-054663
http://www.ncbi.nlm.nih.gov/pubmed/34851422?tool=bestpractice.com
[46]Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010 Jun 25;59(RR-5):1-25.
https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5905a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/20577159?tool=bestpractice.com
An IGRA is preferred in individuals with a history of bacille Calmette-Guérin vaccination due to superior specificity.[45]Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008 Aug 5;149(3):177-84.
http://www.ncbi.nlm.nih.gov/pubmed/18593687?tool=bestpractice.com
In addition, IGRA is preferred for testing persons from groups that historically have low rates of returning to have TSTs read.
Targeted testing for latent TB infection is recommended by the US Centers for Disease Control and Prevention/American Thoracic Society as part of strategic control and reduction of TB. High-risk groups include people with HIV, intravenous drug users, healthcare workers who serve high-risk populations, and contacts of individuals with pulmonary TB.[12]Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):e1-33.
https://academic.oup.com/cid/article/64/2/e1/2629583
http://www.ncbi.nlm.nih.gov/pubmed/27932390?tool=bestpractice.com
Testing for latent TB infection should also be performed in patients prior to tumor necrosis factor-alpha antagonist therapy.[47]Doherty SD, Van Voorhees A, Lebwohl MG, et al. National Psoriasis Foundation consensus statement on screening for latent tuberculosis infection in patients with psoriasis treated with systemic and biologic agents. J Am Acad Dermatol. 2008 Aug;59(2):209-17.
http://www.ncbi.nlm.nih.gov/pubmed/18485527?tool=bestpractice.com
TB antigen-based skin tests (TBSTs) are a new class of tests that have been developed to measure the cell-mediated immunologic response to M tuberculosis specific antigens. The WHO recommends that TBSTs may be used to test for latent TB infection, reporting that the diagnostic accuracy of TBSTs is similar to that of IGRAs and greater than that of the TST.[48]World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: tests for TB infection. Sep 2022 [internet publication].
https://www.who.int/publications/i/item/9789240056084

 ]
[
]
[  ]
The World Health Organization (WHO) recommends that LF-LAM can be used to assist in the diagnosis of active TB in HIV-positive adults, adolescents, and children.[32] This approach is supported by another Cochrane review, which found reductions in mortality and an increase in treatment initiation with use of LF-LAM in inpatient and outpatient settings.[40] Culture would still be required for drug susceptibility testing (DST).
]
The World Health Organization (WHO) recommends that LF-LAM can be used to assist in the diagnosis of active TB in HIV-positive adults, adolescents, and children.[32] This approach is supported by another Cochrane review, which found reductions in mortality and an increase in treatment initiation with use of LF-LAM in inpatient and outpatient settings.[40] Culture would still be required for drug susceptibility testing (DST).