Abdominal aortic aneurysm
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
ruptured AAA
standard resuscitation measures
Standard resuscitation measures should be initiated immediately. These include: airway management (supplemental oxygen or endotracheal intubation and assisted ventilation if the patient is unconscious); securing intravenous access (central venous catheter); arterial catheter and urinary catheter; ensuring blood product availability (packed red cells, platelets, and fresh frozen plasma) and transfusing for resuscitation, severe anemia, and coagulopathy; and notifying anesthetic, intensive care unit (ICU), and operating teams.
Aggressive fluid replacement may cause dilutional and hypothermic coagulopathy and secondary clot disruption from increased blood flow, increased perfusion pressure, and decreased blood viscosity, thereby exacerbating bleeding.[119]Roberts K, Revell M, Youssef H, et al. Hypotensive resuscitation in patients with ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2006 Apr;31(4):339-44. http://www.ncbi.nlm.nih.gov/pubmed/16388972?tool=bestpractice.com [120]Ohki T, Veith FJ. Endovascular grafts and other image-guided catheter-based adjuncts to improve the treatment of ruptured aortoiliac aneurysms. Ann Surg. 2000 Oct;232(4):466-79. http://www.ncbi.nlm.nih.gov/pubmed/10998645?tool=bestpractice.com A target systolic blood pressure (SBP) of 50 to 70 mmHg and withholding fluids is advocated preoperatively.[119]Roberts K, Revell M, Youssef H, et al. Hypotensive resuscitation in patients with ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2006 Apr;31(4):339-44. http://www.ncbi.nlm.nih.gov/pubmed/16388972?tool=bestpractice.com [120]Ohki T, Veith FJ. Endovascular grafts and other image-guided catheter-based adjuncts to improve the treatment of ruptured aortoiliac aneurysms. Ann Surg. 2000 Oct;232(4):466-79. http://www.ncbi.nlm.nih.gov/pubmed/10998645?tool=bestpractice.com The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend permissive hypotension to reduce bleeding.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com However, recommended targets vary and there is no consensus among global guideline groups.
How to insert a tracheal tube in an adult using a laryngoscope.
How to use bag-valve-mask apparatus to deliver ventilatory support to adults. Video demonstrates the two-person technique.
urgent surgical repair
Treatment recommended for ALL patients in selected patient group
The American College of Cardiology/American Heart Association (ACC/AHA) recommend computed tomography (CT) imaging in patients presenting with ruptured AAA who are hemodynamically stable to evaluate whether the AAA is amenable to endovascular repair.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com This recommendation is supported by results from the IMPROVE multicenter randomized controlled trial, which suggest that for most patients, confirmatory CT did not add significant delays to treatment and facilitated appropriate preoperative planning.[108]Powell JT, Hinchcliffe RJ, Thompson MM, et al; IMPROVE Trial Investigators. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg. 2014 Feb;101(3):216-24. https://bjssjournals.onlinelibrary.wiley.com/doi/full/10.1002/bjs.9410 http://www.ncbi.nlm.nih.gov/pubmed/24469620?tool=bestpractice.com
If the anatomy is suitable, the ACC/AHA recommend endovascular repair over open repair to reduce the risk of morbidity and mortality.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com In patients with confirmed ruptured AAA, 3-year mortality was lower among those randomized to endovascular aneurysm repair (EVAR) than to an open repair strategy (48% vs. 56%; hazard ratio [HR] 0.57, 95% CI 0.36 to 0.90).[109]IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017 Nov 14;359:j4859. https://www.bmj.com/content/359/bmj.j4859.long http://www.ncbi.nlm.nih.gov/pubmed/29138135?tool=bestpractice.com The difference between treatment groups was no longer evident after 7 years of follow-up (HR 0.92, 95% CI 0.75 to 1.13). Re-intervention rates were not significantly different between the randomized groups at 3 years (HR 1.02, 95% CI 0.79 to 1.32).[109]IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017 Nov 14;359:j4859. https://www.bmj.com/content/359/bmj.j4859.long http://www.ncbi.nlm.nih.gov/pubmed/29138135?tool=bestpractice.com There is some evidence to suggest that an endovascular strategy for repair of ruptured AAA may reduce mortality more effectively in women than in men.[109]IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017 Nov 14;359:j4859. https://www.bmj.com/content/359/bmj.j4859.long http://www.ncbi.nlm.nih.gov/pubmed/29138135?tool=bestpractice.com [110]Sweeting MJ, Balm R, Desgranges P, et al; Ruptured Aneurysm Trialists. Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg. 2015 Sep;102(10):1229-39. https://bjssjournals.onlinelibrary.wiley.com/doi/full/10.1002/bjs.9852 http://www.ncbi.nlm.nih.gov/pubmed/26104471?tool=bestpractice.com
There is some evidence to suggest that mode of anesthesia for operative repair of AAA affects outcomes.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [111]Armstrong RA, Squire YG, Rogers CA, et al. Type of anesthesia for endovascular abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth. 2019 Feb;33(2):462-71. http://www.ncbi.nlm.nih.gov/pubmed/30342821?tool=bestpractice.com In 2024, the European Society for Vascular Surgery (ESVS) issued a weak recommendation favoring local anesthesia over general anesthesia in elective settings, based on potential reduction in procedure time, ICU admissions, and postoperative hospital stay.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [112]Liu Y, Wang T, Zhao J, et al. Influence of anesthetic techniques on perioperative outcomes after endovascular aneurysm repair. Ann Vasc Surg. 2021 May;73:375-84. http://www.ncbi.nlm.nih.gov/pubmed/33383135?tool=bestpractice.com [113]Zottola ZR, Kruger JL, Kong DS, et al. Locoregional anesthesia is associated with reduced hospital stay and need for intensive care unit care of elective endovascular aneurysm repair patients in the Vascular Quality Initiative. J Vasc Surg. 2023 Apr;77(4):1061-9. https://www.jvascsurg.org/article/S0741-5214(22)02534-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/36400363?tool=bestpractice.com [114]Kothandan H, Haw Chieh GL, Khan SA, et al. Anesthetic considerations for endovascular abdominal aortic aneurysm repair. Ann Card Anaesth. 2016 Jan-Mar;19(1):132-41. https://pmc.ncbi.nlm.nih.gov/articles/PMC4900395 http://www.ncbi.nlm.nih.gov/pubmed/26750684?tool=bestpractice.com The IMPROVE multicenter randomized controlled trial detected a significantly reduced 30-day mortality in patients who had EVAR under local anesthesia alone compared with general anesthesia (adjusted OR 0.27, 0.1 to 0.7).[108]Powell JT, Hinchcliffe RJ, Thompson MM, et al; IMPROVE Trial Investigators. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg. 2014 Feb;101(3):216-24. https://bjssjournals.onlinelibrary.wiley.com/doi/full/10.1002/bjs.9410 http://www.ncbi.nlm.nih.gov/pubmed/24469620?tool=bestpractice.com A separate meta-analysis comparing mode of anesthesia for endovascular repair of ruptured AAA replicated these findings or improved outcomes with EVAR under local anesthesia.[115]Harky A, Ahmad MU, Santoro G, et al. Local versus general anesthesia in nonemergency endovascular abdominal aortic aneurysm repair: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2020 Apr;34(4):1051-9. http://www.ncbi.nlm.nih.gov/pubmed/31473112?tool=bestpractice.com However, another systematic review did not show any mortality benefit with local anesthesia, but did demonstrate shorter hospital stays.[116]Deng J, Liu J, Rong D, et al. A meta-analysis of locoregional anesthesia versus general anesthesia in endovascular repair of ruptured abdominal aortic aneurysm. J Vasc Surg. 2021 Feb;73(2):700-10. https://www.doi.org/10.1016/j.jvs.2020.08.112 http://www.ncbi.nlm.nih.gov/pubmed/32882348?tool=bestpractice.com Data from the UK’s National Vascular Registry (9783 patients who received an elective, standard infrarenal EVAR; general anesthetic, n = 7069; regional anesthetic, n = 2347; local anesthetic, n = 367) showed a lower 30 day mortality rate after regional versus general anesthesia.[117]Dovell G, Rogers CA, Armstrong R, et al. The effect of mode of anaesthesia on outcomes after elective endovascular repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2020 May;59(5):729-38. https://www.ejves.com/article/S1078-5884(20)30118-0/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32291124?tool=bestpractice.com The international multicenter Endurant Stent Graft Natural Selection Global Post-Market Registry (ENGAGE) study examined the outcomes of 1231 patients undergoing EVAR under general (62% of patients), regional (27%), and local (11%) anesthesia.[118]Broos PP, Stokmans RA, Cuypers PW, et al. Effects of anesthesia type on perioperative outcome after endovascular aneurysm repair. J Endovasc Ther. 2015 Oct;22(5):770-7. http://www.ncbi.nlm.nih.gov/pubmed/26276553?tool=bestpractice.com The type of anesthesia had no influence on perioperative mortality or morbidity but the use of local or regional anesthesia during EVAR appeared to be beneficial in decreasing procedure time, need for ICU admission, and duration of postoperative hospital stay.[118]Broos PP, Stokmans RA, Cuypers PW, et al. Effects of anesthesia type on perioperative outcome after endovascular aneurysm repair. J Endovasc Ther. 2015 Oct;22(5):770-7. http://www.ncbi.nlm.nih.gov/pubmed/26276553?tool=bestpractice.com
perioperative antibiotic therapy
Treatment recommended for ALL patients in selected patient group
Antibiotic therapy is indicated for patients undergoing emergency repair of ruptured AAA to cover gram-positive and gram-negative organisms and prevent graft infection.
Broad-spectrum antibiotic coverage is tailored to patient clinical presentation and cultures, and in accordance with local protocols.
treatment of infectious/inflammatory cause
Treatment recommended for SOME patients in selected patient group
Once the patient is stable and urgent surgical repair for the rupture has been prioritized, infectious or inflammatory etiology should be addressed.
If the patient has a suspected infectious aneurysm, early diagnosis and prompt treatment is essential to improve outcomes.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com Extensive debridement is often needed during urgent surgical repair in these patients. There is a high risk of secondary infective complications and further surgery may be needed for new infectious lesions. Intraoperative cultures should be taken to accurately guide subsequent antibiotic therapy; however, empirical antibiotics are often administered, as peripheral blood cultures and surgical specimen cultures are negative in a large proportion of patients.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Prolonged antibiotic therapy (from 4-6 weeks duration to lifelong) may be indicated depending on the specific pathogen, the type of operative repair, and the patient's immunological state.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
Inflammatory aortitis (caused by, for example, Takayasu arteritis or giant cell arteritis) is treated with high-dose corticosteroids and surgery.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [203]Ben Jmaà H, Karray R, Jmal H, et al. Surgical and endoluminal management of the inflammatory aortitis: a Tunisian center experience [in French]. J Med Vasc. 2017 Jul;42(4):213-20. http://www.ncbi.nlm.nih.gov/pubmed/28705339?tool=bestpractice.com
symptomatic, but not ruptured AAA
urgent surgical repair
In patients with symptomatic aortic aneurysm, urgent repair is indicated regardless of diameter.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [78]National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. Mar 2020 [internet publication]. https://www.nice.org.uk/guidance/ng156 [102]Mazzolai L, Teixido-Tura G, Lanzi S, et al. 2024 ESC guidelines for the management of peripheral arterial and aortic diseases. Eur Heart J. 2024 Sep 29;45(36):3538-700. https://academic.oup.com/eurheartj/article/45/36/3538/7738955 The development of new or worsening pain may herald aneurysm expansion and impending rupture. Symptomatic, nonruptured aneurysm is, therefore, best treated urgently.[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com Under some circumstances, intervention may be delayed for several hours to optimize conditions to ensure successful repair; these patients should be closely monitored in the intensive care unit.[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com
Endovascular aneurysm repair (EVAR) is increasingly used in the management of patients with symptomatic AAA.[125]De Martino RR, Nolan BW, Goodney PP, Chang CK, et al; Vascular Study Group of Northern New England. Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg. 2010 Jul;52(1):5-12.e1. https://www.jvascsurg.org/article/S0741-5214(10)00259-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/20471771?tool=bestpractice.com [126]Chandra V, Trang K, Virgin-Downey W, et al. Management and outcomes of symptomatic abdominal aortic aneurysms during the past 20 years. J Vasc Surg. 2017 Dec;66(6):1679-85. http://www.ncbi.nlm.nih.gov/pubmed/28619644?tool=bestpractice.com In observational studies, short-term all-cause mortality rates did not differ between endovascular and open repair of symptomatic AAA.[125]De Martino RR, Nolan BW, Goodney PP, Chang CK, et al; Vascular Study Group of Northern New England. Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg. 2010 Jul;52(1):5-12.e1. https://www.jvascsurg.org/article/S0741-5214(10)00259-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/20471771?tool=bestpractice.com [126]Chandra V, Trang K, Virgin-Downey W, et al. Management and outcomes of symptomatic abdominal aortic aneurysms during the past 20 years. J Vasc Surg. 2017 Dec;66(6):1679-85. http://www.ncbi.nlm.nih.gov/pubmed/28619644?tool=bestpractice.com [127]Ten Bosch JA, Willigendael EM, Kruidenier LM, et al. Early and mid-term results of a prospective observational study comparing emergency endovascular aneurysm repair with open surgery in both ruptured and unruptured acute abdominal aortic aneurysms. Vascular. 2012 Apr;20(2):72-80. http://www.ncbi.nlm.nih.gov/pubmed/22454547?tool=bestpractice.com
preoperative cardiovascular risk reduction
Treatment recommended for ALL patients in selected patient group
Addressing modifiable cardiovascular risk factors preoperatively improves long-term survival after AAA repair.[194]Khashram M, Williman JA, Hider PN, et al. Management of modifiable vascular risk factors improves late survival following abdominal aortic aneurysm repair: a systematic review and meta-analysis. Ann Vasc Surg. 2017 Feb;39:301-11. http://www.ncbi.nlm.nih.gov/pubmed/27666804?tool=bestpractice.com
Preoperative exercise training reduced postsurgical cardiac complications in a small randomized controlled trial (RCT) of patients undergoing open or endovascular AAA repair, though a Cochrane review and a separate systematic review of prehabilitation (exercise training) prior to AAA surgery did not show any outcome benefit.[195]Barakat HM, Shahin Y, Khan JA, et al. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair: a randomized controlled trial. Ann Surg. 2016 Jul;264(1):47-53. http://www.ncbi.nlm.nih.gov/pubmed/26756766?tool=bestpractice.com [196]Fenton C, Tan AR, Abaraogu UO, et al. Prehabilitation exercise therapy before elective abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2021 Jul 8;7(7):CD013662. https://www.doi.org/10.1002/14651858.CD013662.pub2 http://www.ncbi.nlm.nih.gov/pubmed/34236703?tool=bestpractice.com [197]Bonner RJ, Wallace T, Jones AD, et al. The content of pre-habilitative interventions for patients undergoing repair of abdominal aortic aneurysms and their effect on post-operative outcomes: a systematic review. Eur J Vasc Endovasc Surg. 2021 May;61(5):756-65. https://www.doi.org/10.1016/j.ejvs.2021.01.043 http://www.ncbi.nlm.nih.gov/pubmed/33678532?tool=bestpractice.com While preoperative exercise training may be beneficial for patients undergoing AAA repair, further investigation with RCTs is needed before it can be recommended more widely.[198]Wee IJY, Choong AMTL. A systematic review of the impact of preoperative exercise for patients with abdominal aortic aneurysm. J Vasc Surg. 2020 Jun;71(6):2123-31.e1. https://www.doi.org/10.1016/j.jvs.2018.09.039 http://www.ncbi.nlm.nih.gov/pubmed/30606665?tool=bestpractice.com
Perioperative statin use slows aneurysm growth, reduces risk of rupture and, reduces mortality from AAA repair or ruptured AAA.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Statins should be started at least 1 month before surgery to reduce cardiovascular morbidity and mortality, and continued indefinitely.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [139]Risum Ø, Sandven I, Sundhagen JO, et al. Editor's choice - effect of statins on total mortality in abdominal aortic aneurysm repair: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2021 Jan;61(1):114-20. https://www.doi.org/10.1016/j.ejvs.2020.08.007 http://www.ncbi.nlm.nih.gov/pubmed/32928667?tool=bestpractice.com
Patients with AAAs are at increased risk of major adverse cardiovascular events. There is limited evidence, but in the absence of any contraindication, patients with AAA should receive single antiplatelet therapy (aspirin or clopidogrel).[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [140]Aboyans V, Bauersachs R, Mazzolai L, et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: a consensus document from the ESC working group on aorta and peripheral vascular diseases, the ESC working group on thrombosis, and the ESC working group on cardiovascular pharmacotherapy. Eur Heart J. 2021 Oct 14;42(39):4013-24. https://www.doi.org/10.1093/eurheartj/ehab390 http://www.ncbi.nlm.nih.gov/pubmed/34279602?tool=bestpractice.com This should be continued during the perioperative period.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
Hypertension should be controlled to reduce cardiovascular morbidity and mortality.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
Initiation of beta blockers is not recommended prior to AAA repair.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com However, a beta-blocker can be continued if a patient is already taking this at an appropriate dose.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com Large trials where beta-blockade was started a few days before surgery have indicated no benefit, or even harm, from perioperative beta-blockade.[199]Brady AR, Gibbs JS, Greenhalgh RM, et al; POBBLE Trial Investigators. Perioperative beta-blockade (Pobble) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg. 2005 Apr;41(4):602-9. https://www.jvascsurg.org/article/S0741-5214(05)00189-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/15874923?tool=bestpractice.com [200]Devereaux PJ, Yang H, Yusuf S, et al; POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008 May 31;371(9627):1839-47. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(08)60601-7/fulltext http://www.ncbi.nlm.nih.gov/pubmed/18479744?tool=bestpractice.com [201]Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006 Nov;152(5):983-90. http://www.ncbi.nlm.nih.gov/pubmed/17070177?tool=bestpractice.com
perioperative antibiotic therapy
Treatment recommended for ALL patients in selected patient group
Perioperative antibiotic therapy is given. Broad-spectrum antibiotic coverage is necessary, in accordance with local protocols.
treatment of infectious/inflammatory cause
Treatment recommended for SOME patients in selected patient group
Once the patient is stable and urgent surgical repair for symptomatic AAA has been prioritized, infectious or inflammatory etiology should be addressed.
If the patient has a suspected infectious aneurysm, early diagnosis and prompt treatment is essential to improve outcomes.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com Extensive debridement is often needed during urgent surgical repair in these patients. There is a high risk of secondary infective complications and further surgery may be needed for new infectious lesions. Intraoperative cultures should be taken to accurately guide subsequent antibiotic therapy; however, empirical antibiotics are often administered, as peripheral blood cultures and surgical specimen cultures are negative in a large proportion of patients.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Prolonged antibiotic therapy (from 4-6 weeks duration to lifelong) may be indicated depending on the specific pathogen, the type of operative repair, and the patient's immunological state.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
Inflammatory aortitis (caused by, for example, Takayasu arteritis or giant cell arteritis) is treated with high-dose corticosteroids and surgery.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [203]Ben Jmaà H, Karray R, Jmal H, et al. Surgical and endoluminal management of the inflammatory aortitis: a Tunisian center experience [in French]. J Med Vasc. 2017 Jul;42(4):213-20. http://www.ncbi.nlm.nih.gov/pubmed/28705339?tool=bestpractice.com
incidental finding: small asymptomatic AAA
surveillance
For AAA detected as an incidental finding, surveillance is preferred to repair until the theoretical risk of rupture exceeds the estimated risk of operative mortality.[4]Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019 Dec 10;322(22):2211-8. https://jamanetwork.com/journals/jama/fullarticle/2757234 http://www.ncbi.nlm.nih.gov/pubmed/31821437?tool=bestpractice.com
Early open surgery for the treatment of smaller AAAs does not reduce all-cause or AAA-specific mortality.[4]Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019 Dec 10;322(22):2211-8.
https://jamanetwork.com/journals/jama/fullarticle/2757234
http://www.ncbi.nlm.nih.gov/pubmed/31821437?tool=bestpractice.com
[129]Ulug P, Powell JT, Martinez MA, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD001835.
https://www.doi.org/10.1002/14651858.CD001835.pub5
http://www.ncbi.nlm.nih.gov/pubmed/32609382?tool=bestpractice.com
[  ]
How does immediate surgery compare with surveillance in people with asymptomatic abdominal aortic aneurysms (AAAs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3227/fullShow me the answer One systematic review (4 trials, 3314 participants) found high-quality evidence to demonstrate that immediate repair of small AAA (4.0 cm to 5.5 cm) did not improve long-term survival compared with surveillance (adjusted hazard ratio 0.88, 95% CI 0.75 to 1.02, mean follow-up 10 years).[129]Ulug P, Powell JT, Martinez MA, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD001835.
https://www.doi.org/10.1002/14651858.CD001835.pub5
http://www.ncbi.nlm.nih.gov/pubmed/32609382?tool=bestpractice.com
The lack of benefit attributable to immediate surgery was consistent regardless of patient age, diameter of small aneurysm, and whether repair was endovascular or open.[129]Ulug P, Powell JT, Martinez MA, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD001835.
https://www.doi.org/10.1002/14651858.CD001835.pub5
http://www.ncbi.nlm.nih.gov/pubmed/32609382?tool=bestpractice.com
]
How does immediate surgery compare with surveillance in people with asymptomatic abdominal aortic aneurysms (AAAs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3227/fullShow me the answer One systematic review (4 trials, 3314 participants) found high-quality evidence to demonstrate that immediate repair of small AAA (4.0 cm to 5.5 cm) did not improve long-term survival compared with surveillance (adjusted hazard ratio 0.88, 95% CI 0.75 to 1.02, mean follow-up 10 years).[129]Ulug P, Powell JT, Martinez MA, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD001835.
https://www.doi.org/10.1002/14651858.CD001835.pub5
http://www.ncbi.nlm.nih.gov/pubmed/32609382?tool=bestpractice.com
The lack of benefit attributable to immediate surgery was consistent regardless of patient age, diameter of small aneurysm, and whether repair was endovascular or open.[129]Ulug P, Powell JT, Martinez MA, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD001835.
https://www.doi.org/10.1002/14651858.CD001835.pub5
http://www.ncbi.nlm.nih.gov/pubmed/32609382?tool=bestpractice.com
Surgical referral of smaller AAA is usually reserved for rapid growth, or once the threshold diameter for aneurysm repair is reached on repeated ultrasonography.[4]Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019 Dec 10;322(22):2211-8. https://jamanetwork.com/journals/jama/fullarticle/2757234 http://www.ncbi.nlm.nih.gov/pubmed/31821437?tool=bestpractice.com
However, in patients with an underlying genetic cause or connective tissue disorder, the threshold diameter for considering repair should be individualized, depending on anatomic features and underlying genetics (rupture risk is higher at smaller aortic diameters in some conditions, and surgical repair is more challenging in certain disorders owing to the increased arterial wall fragility and anatomy).[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
aggressive cardiovascular risk management
Treatment recommended for ALL patients in selected patient group
Addressing modifiable cardiovascular risk factors preoperatively improves long-term survival after AAA repair.[194]Khashram M, Williman JA, Hider PN, et al. Management of modifiable vascular risk factors improves late survival following abdominal aortic aneurysm repair: a systematic review and meta-analysis. Ann Vasc Surg. 2017 Feb;39:301-11. http://www.ncbi.nlm.nih.gov/pubmed/27666804?tool=bestpractice.com
Patients should be encouraged to stop smoking and offered drug therapy (nicotine-replacement therapy, nortriptyline, and bupropion) or counseling to assist with this if needed.[1]Dehlin JM, Upchurch GR. Management of abdominal aortic aneurysms. Curr Treat Options Cardiovasc Med. 2005 Jun;7(2):119-30.
http://www.ncbi.nlm.nih.gov/pubmed/15935120?tool=bestpractice.com
[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482.
https://www.doi.org/10.1161/CIR.0000000000001106
http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
[13]Zankl AR, Schumacher H, Krumsdorf U, et al. Pathology, natural history and treatment of abdominal aortic aneurysms. Clin Res Cardiol. 2007 Mar;96(3):140-51.
http://www.ncbi.nlm.nih.gov/pubmed/17180573?tool=bestpractice.com
[15]Singh K, Bønaa H, Jacobsen BK, et al. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001 Aug 1;154(3):236-44.
https://academic.oup.com/aje/article/154/3/236/125840
http://www.ncbi.nlm.nih.gov/pubmed/11479188?tool=bestpractice.com
[22]Lederle FA, Johnson GR, Wilson SE, et al; Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Intern Med. 1997 Mar 15;126(6):441-9.
http://www.ncbi.nlm.nih.gov/pubmed/9072929?tool=bestpractice.com
[23]Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999 Dec;30(6):1099-105.
http://www.ncbi.nlm.nih.gov/pubmed/10587395?tool=bestpractice.com
[134]Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018 May 31;5:CD000146.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000146.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/29852054?tool=bestpractice.com
[135]Rigotti NA, Clair C, Munafò MR, et al. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012 May 16;(5):CD001837.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001837.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/22592676?tool=bestpractice.com
[136]Howes S, Hartmann-Boyce J, Livingstone-Banks J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2020 Apr 22;(4):CD000031.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000031.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/32319681?tool=bestpractice.com
[  ]
What are the effects of adding bupropion or fluoxetine to other treatments compared with using other treatments alone for people trying to quit smoking?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4337/fullShow me the answer
]
What are the effects of adding bupropion or fluoxetine to other treatments compared with using other treatments alone for people trying to quit smoking?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4337/fullShow me the answer
Preoperative exercise training reduced postsurgical cardiac complications in a small randomized controlled trial (RCT) of patients undergoing open or endovascular AAA repair, though a Cochrane review and a separate systematic review of prehabilitation (exercise training) prior to AAA surgery did not show any outcome benefit.[195]Barakat HM, Shahin Y, Khan JA, et al. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair: a randomized controlled trial. Ann Surg. 2016 Jul;264(1):47-53. http://www.ncbi.nlm.nih.gov/pubmed/26756766?tool=bestpractice.com [196]Fenton C, Tan AR, Abaraogu UO, et al. Prehabilitation exercise therapy before elective abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2021 Jul 8;7(7):CD013662. https://www.doi.org/10.1002/14651858.CD013662.pub2 http://www.ncbi.nlm.nih.gov/pubmed/34236703?tool=bestpractice.com [197]Bonner RJ, Wallace T, Jones AD, et al. The content of pre-habilitative interventions for patients undergoing repair of abdominal aortic aneurysms and their effect on post-operative outcomes: a systematic review. Eur J Vasc Endovasc Surg. 2021 May;61(5):756-65. https://www.doi.org/10.1016/j.ejvs.2021.01.043 http://www.ncbi.nlm.nih.gov/pubmed/33678532?tool=bestpractice.com While preoperative exercise training may be beneficial for patients undergoing AAA repair, further investigation with RCTs is needed before it can be recommended more widely.[198]Wee IJY, Choong AMTL. A systematic review of the impact of preoperative exercise for patients with abdominal aortic aneurysm. J Vasc Surg. 2020 Jun;71(6):2123-31.e1. https://www.doi.org/10.1016/j.jvs.2018.09.039 http://www.ncbi.nlm.nih.gov/pubmed/30606665?tool=bestpractice.com
Perioperative statin use slows aneurysm growth, reduces risk of rupture and, reduces mortality from AAA repair or ruptured AAA.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Statins should be started at least 1 month before surgery to reduce cardiovascular morbidity and mortality, and continued indefinitely.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [139]Risum Ø, Sandven I, Sundhagen JO, et al. Editor's choice - effect of statins on total mortality in abdominal aortic aneurysm repair: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2021 Jan;61(1):114-20. https://www.doi.org/10.1016/j.ejvs.2020.08.007 http://www.ncbi.nlm.nih.gov/pubmed/32928667?tool=bestpractice.com
Patients with AAAs are at increased risk of major adverse cardiovascular events. There is limited evidence, but in the absence of any contraindication, patients with AAA should receive single antiplatelet therapy (aspirin or clopidogrel).[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [140]Aboyans V, Bauersachs R, Mazzolai L, et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: a consensus document from the ESC working group on aorta and peripheral vascular diseases, the ESC working group on thrombosis, and the ESC working group on cardiovascular pharmacotherapy. Eur Heart J. 2021 Oct 14;42(39):4013-24. https://www.doi.org/10.1093/eurheartj/ehab390 http://www.ncbi.nlm.nih.gov/pubmed/34279602?tool=bestpractice.com This should be continued during the perioperative period.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
Hypertension should be controlled to reduce cardiovascular morbidity and mortality.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
treatment of infectious/inflammatory cause
Treatment recommended for SOME patients in selected patient group
Infectious or inflammatory etiology should be addressed.
If the patient has a suspected infectious aneurysm, early diagnosis and prompt treatment with antibiotics and urgent surgical repair is essential to improve outcomes.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com Extensive debridement is often needed during urgent surgical repair in these patients. There is a high risk of secondary infective complications and further surgery may be needed for new infectious lesions. Intraoperative cultures should be taken to accurately guide subsequent antibiotic therapy; however, empirical antibiotics are often administered, as peripheral blood cultures and surgical specimen cultures are negative in a large proportion of patients.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Prolonged antibiotic therapy (from 4-6 weeks duration to lifelong) may be indicated depending on the specific pathogen, the type of operative repair, and the patient's immunological state.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
Inflammatory aortitis (caused by, for example, Takayasu arteritis or giant cell arteritis) is treated with high-dose corticosteroids and surgery.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [203]Ben Jmaà H, Karray R, Jmal H, et al. Surgical and endoluminal management of the inflammatory aortitis: a Tunisian center experience [in French]. J Med Vasc. 2017 Jul;42(4):213-20. http://www.ncbi.nlm.nih.gov/pubmed/28705339?tool=bestpractice.com
incidental finding: large asymptomatic AAA
elective surgical repair
Generally, repair is indicated in patients with large asymptomatic AAA (e.g., with a diameter >5.5 cm in men or >5.0 cm in women in the US, although treatment decisions based on greater size may differ in other countries).[78]National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. Mar 2020 [internet publication]. https://www.nice.org.uk/guidance/ng156 Repair of aneurysms ≥5.5 cm offers a survival advantage.[1]Dehlin JM, Upchurch GR. Management of abdominal aortic aneurysms. Curr Treat Options Cardiovasc Med. 2005 Jun;7(2):119-30. http://www.ncbi.nlm.nih.gov/pubmed/15935120?tool=bestpractice.com [76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com [104]UK Small Aneurysm Trial Participants. Mortality results for randomized controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998 Nov 21;352(9141):1649-55. http://www.ncbi.nlm.nih.gov/pubmed/9853436?tool=bestpractice.com [105]Powell JT, Brady AR, Brown LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002 May 9;346(19):1445-52. https://www.nejm.org/doi/full/10.1056/NEJMoa013527 http://www.ncbi.nlm.nih.gov/pubmed/12000814?tool=bestpractice.com [106]Powell JT, Brown LC, Forbes JF, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg. 2007 Jun;94(6):702-8. http://www.ncbi.nlm.nih.gov/pubmed/17514693?tool=bestpractice.com
Decisions regarding repair should be individualized, taking account of patient preference, patient age, sex, perioperative risk factors, and anatomic risk factors. Care should be taken to evaluate patient quality of life, and careful counseling undertaken regarding the risks of surgery and subsequent quality of life. A shared decision making approach taking into account the risks and benefits of the procedures is recommended.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [129]Ulug P, Powell JT, Martinez MA, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD001835. https://www.doi.org/10.1002/14651858.CD001835.pub5 http://www.ncbi.nlm.nih.gov/pubmed/32609382?tool=bestpractice.com
Data suggest that in patients with large AAAs (≥5.5 cm) undergoing elective repair, EVAR is equivalent to open repair in terms of overall survival, although the rate of secondary interventions is higher for EVAR.[141]Greenhalgh RM, Brown LC, Powell JT, et al; United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010 May 20;362(20):1863-71. http://www.ncbi.nlm.nih.gov/pubmed/20382983?tool=bestpractice.com [142]Amato B, Fugetto F, Compagna R, et al. Endovascular repair versus open repair in the treatment of ruptured aortic aneurysms: a systematic review. Minerva Chir. 2019 Dec;74(6):472-80. http://www.ncbi.nlm.nih.gov/pubmed/29806754?tool=bestpractice.com EVAR reduces AAA-related mortality (but not longer-term overall survival) in patients with large AAA (≥5.5 cm) who are unsuitable for open repair.[143]Greenhalgh RM, Brown LC, Powell JT, et al; United Kingdom EVAR Trial Investigators. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med. 2010 May 20;362(20):1872-80. http://www.ncbi.nlm.nih.gov/pubmed/20382982?tool=bestpractice.com
Post repair, larger AAAs appear to be associated with worse late survival than smaller aneurysms (pooled hazard ratio 1.14 per 1-cm increase in AAA diameter, 95% CI 1.09 to 1.18; 12.0- to 91.2-month follow-up).[144]Khashram M, Hider PN, Williman JA, et al. Does the diameter of abdominal aortic aneurysm influence late survival following abdominal aortic aneurysm repair? A systematic review and meta-analysis. Vascular. 2016 Dec;24(6):658-67. http://www.ncbi.nlm.nih.gov/pubmed/27189809?tool=bestpractice.com The association is more pronounced with EVAR than with open repair.
For patients with a complex AAA and standard surgical risk, open or EVAR should be considered based on fitness, anatomy, and patient preference. For patients with a complex AAA and high surgical risk, EVAR with fenestrated and branched technologies should be considered as first-line therapy. Fenestrated and branched endografts have become the treatment of choice of complex AAAs in most high volume centers.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com These procedures are viable alternatives to open repair for juxtarenal and suprarenal AAA, or for those with AAA where a short or diseased neck precludes conventional repair.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
preoperative cardiovascular risk reduction
Treatment recommended for ALL patients in selected patient group
Addressing modifiable cardiovascular risk factors preoperatively improves long-term survival after AAA repair.[194]Khashram M, Williman JA, Hider PN, et al. Management of modifiable vascular risk factors improves late survival following abdominal aortic aneurysm repair: a systematic review and meta-analysis. Ann Vasc Surg. 2017 Feb;39:301-11. http://www.ncbi.nlm.nih.gov/pubmed/27666804?tool=bestpractice.com
Patients should be encouraged to stop smoking and offered drug therapy (nicotine-replacement therapy, nortriptyline, and bupropion) or counseling to assist with this if needed.[1]Dehlin JM, Upchurch GR. Management of abdominal aortic aneurysms. Curr Treat Options Cardiovasc Med. 2005 Jun;7(2):119-30.
http://www.ncbi.nlm.nih.gov/pubmed/15935120?tool=bestpractice.com
[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482.
https://www.doi.org/10.1161/CIR.0000000000001106
http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
[13]Zankl AR, Schumacher H, Krumsdorf U, et al. Pathology, natural history and treatment of abdominal aortic aneurysms. Clin Res Cardiol. 2007 Mar;96(3):140-51.
http://www.ncbi.nlm.nih.gov/pubmed/17180573?tool=bestpractice.com
[15]Singh K, Bønaa H, Jacobsen BK, et al. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001 Aug 1;154(3):236-44.
https://academic.oup.com/aje/article/154/3/236/125840
http://www.ncbi.nlm.nih.gov/pubmed/11479188?tool=bestpractice.com
[22]Lederle FA, Johnson GR, Wilson SE, et al; Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Intern Med. 1997 Mar 15;126(6):441-9.
http://www.ncbi.nlm.nih.gov/pubmed/9072929?tool=bestpractice.com
[23]Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999 Dec;30(6):1099-105.
http://www.ncbi.nlm.nih.gov/pubmed/10587395?tool=bestpractice.com
[134]Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018 May 31;5:CD000146.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000146.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/29852054?tool=bestpractice.com
[135]Rigotti NA, Clair C, Munafò MR, et al. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012 May 16;(5):CD001837.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001837.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/22592676?tool=bestpractice.com
[136]Howes S, Hartmann-Boyce J, Livingstone-Banks J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2020 Apr 22;(4):CD000031.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000031.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/32319681?tool=bestpractice.com
[  ]
What are the effects of adding bupropion or fluoxetine to other treatments compared with using other treatments alone for people trying to quit smoking?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4337/fullShow me the answer
]
What are the effects of adding bupropion or fluoxetine to other treatments compared with using other treatments alone for people trying to quit smoking?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4337/fullShow me the answer
Preoperative exercise training reduced postsurgical cardiac complications in a small randomized controlled trial (RCT) of patients undergoing open or endovascular AAA repair, though a Cochrane review and a separate systematic review of prehabilitation (exercise training) prior to AAA surgery did not show any outcome benefit.[195]Barakat HM, Shahin Y, Khan JA, et al. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair: a randomized controlled trial. Ann Surg. 2016 Jul;264(1):47-53. http://www.ncbi.nlm.nih.gov/pubmed/26756766?tool=bestpractice.com [196]Fenton C, Tan AR, Abaraogu UO, et al. Prehabilitation exercise therapy before elective abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2021 Jul 8;7(7):CD013662. https://www.doi.org/10.1002/14651858.CD013662.pub2 http://www.ncbi.nlm.nih.gov/pubmed/34236703?tool=bestpractice.com [197]Bonner RJ, Wallace T, Jones AD, et al. The content of pre-habilitative interventions for patients undergoing repair of abdominal aortic aneurysms and their effect on post-operative outcomes: a systematic review. Eur J Vasc Endovasc Surg. 2021 May;61(5):756-65. https://www.doi.org/10.1016/j.ejvs.2021.01.043 http://www.ncbi.nlm.nih.gov/pubmed/33678532?tool=bestpractice.com While preoperative exercise training may be beneficial for patients undergoing AAA repair, further investigation with RCTs is needed before it can be recommended more widely.[198]Wee IJY, Choong AMTL. A systematic review of the impact of preoperative exercise for patients with abdominal aortic aneurysm. J Vasc Surg. 2020 Jun;71(6):2123-31.e1. https://www.doi.org/10.1016/j.jvs.2018.09.039 http://www.ncbi.nlm.nih.gov/pubmed/30606665?tool=bestpractice.com
Perioperative statin use slows aneurysm growth, reduces risk of rupture and, reduces mortality from AAA repair or ruptured AAA.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Statins should be started at least 1 month before surgery to reduce cardiovascular morbidity and mortality, and continued indefinitely.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [139]Risum Ø, Sandven I, Sundhagen JO, et al. Editor's choice - effect of statins on total mortality in abdominal aortic aneurysm repair: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2021 Jan;61(1):114-20. https://www.doi.org/10.1016/j.ejvs.2020.08.007 http://www.ncbi.nlm.nih.gov/pubmed/32928667?tool=bestpractice.com
Patients with AAAs are at increased risk of major adverse cardiovascular events. There is limited evidence, but in the absence of any contraindication, patients with AAA should receive single antiplatelet therapy (aspirin or clopidogrel).[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [140]Aboyans V, Bauersachs R, Mazzolai L, et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: a consensus document from the ESC working group on aorta and peripheral vascular diseases, the ESC working group on thrombosis, and the ESC working group on cardiovascular pharmacotherapy. Eur Heart J. 2021 Oct 14;42(39):4013-24. https://www.doi.org/10.1093/eurheartj/ehab390 http://www.ncbi.nlm.nih.gov/pubmed/34279602?tool=bestpractice.com This should be continued during the perioperative period.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
Hypertension should be controlled to reduce cardiovascular morbidity and mortality.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
perioperative antibiotic therapy
Treatment recommended for ALL patients in selected patient group
Perioperative antibiotic therapy is given. Broad-spectrum antibiotic coverage is necessary, in accordance with local protocols.
treatment of infectious/inflammatory cause
Treatment recommended for SOME patients in selected patient group
Infectious or inflammatory etiology should be addressed.
If the patient has a suspected infectious aneurysm, early diagnosis and prompt treatment with antibiotics and urgent surgical repair is essential to improve outcomes.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com Extensive debridement is often needed during urgent surgical repair in these patients. There is a high risk of secondary infective complications and further surgery may be needed for new infectious lesions. Intraoperative cultures should be taken to accurately guide subsequent antibiotic therapy; however, empirical antibiotics are often administered, as peripheral blood cultures and surgical specimen cultures are negative in a large proportion of patients.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Prolonged antibiotic therapy (from 4-6 weeks duration to lifelong) may be indicated depending on the specific pathogen, the type of operative repair, and the patient's immunological state.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
Inflammatory aortitis (caused by, for example, Takayasu arteritis or giant cell arteritis) is treated with high-dose corticosteroids and surgery.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [203]Ben Jmaà H, Karray R, Jmal H, et al. Surgical and endoluminal management of the inflammatory aortitis: a Tunisian center experience [in French]. J Med Vasc. 2017 Jul;42(4):213-20. http://www.ncbi.nlm.nih.gov/pubmed/28705339?tool=bestpractice.com
endovascular repair leak requiring treatment
corrective procedure
Endoleak is persistent blood flow outside the graft and within the aneurysm sac.[204]Schurink GW, Aarts NJ, vanBockel JH. Endoleak after stent-graft treatment of abdominal aortic aneurysm: a meta-analysis of clinical studies. Br J Surg. 1999 May;86(5):581-7. http://www.ncbi.nlm.nih.gov/pubmed/10361173?tool=bestpractice.com [205]Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002 May;35(5):1029-35. http://www.ncbi.nlm.nih.gov/pubmed/12021724?tool=bestpractice.com It is not a complication following open repair.
Postoperative surveillance can detect major endoleaks and aneurysm sac expansion. Risk following endovascular aneurysm repair (EVAR) is 24%.[204]Schurink GW, Aarts NJ, vanBockel JH. Endoleak after stent-graft treatment of abdominal aortic aneurysm: a meta-analysis of clinical studies. Br J Surg. 1999 May;86(5):581-7. http://www.ncbi.nlm.nih.gov/pubmed/10361173?tool=bestpractice.com There are five types of endoleak.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
Type I: leak at the attachment site (type IA at the proximal end of the endograft or iliac occluder; type IB at the distal end); usually immediate, but delayed leaks may occur.[Figure caption and citation for the preceding image starts]: Type I endoleak at the distal left iliac anastomosis (leak encircled)University of Michigan/Dr G.R. Upchurch, Departments of Vascular Surgery and Radiology [Citation ends].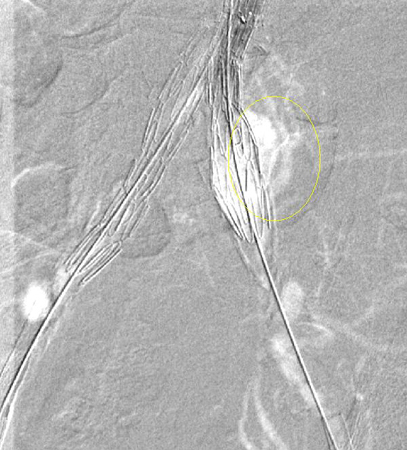 Every effort should be made to repair type I endoleak before completing the procedure (e.g., balloon molding of the proximal seal zone, placement of a proximal cuff, endostaples, liquid embolization).[206]van Schaik TG, Meekel JP, Hoksbergen AWJ, et al. Systematic review of embolization of type I endoleaks using liquid embolic agents. J Vasc Surg. 2021 Sep;74(3):1024-32.
https://www.doi.org/10.1016/j.jvs.2021.03.061
http://www.ncbi.nlm.nih.gov/pubmed/33940072?tool=bestpractice.com
Persistent type IA endoleak may necessitate conversion to open repair, provided the surgical risk is acceptable.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331.
https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2.
https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com
[207]Perini P, Bianchini Massoni C, Mariani E, et al. Systematic review and meta-analysis of the outcome of different treatments for type 1a endoleak after EVAR. Ann Vasc Surg. 2019 Oct;60:435-46.e1.
http://www.ncbi.nlm.nih.gov/pubmed/31200054?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Extension stent graft deployed for the same type I endoleak (encircled)University of Michigan/Dr G.R. Upchurch, Departments of Vascular Surgery and Radiology [Citation ends].
Every effort should be made to repair type I endoleak before completing the procedure (e.g., balloon molding of the proximal seal zone, placement of a proximal cuff, endostaples, liquid embolization).[206]van Schaik TG, Meekel JP, Hoksbergen AWJ, et al. Systematic review of embolization of type I endoleaks using liquid embolic agents. J Vasc Surg. 2021 Sep;74(3):1024-32.
https://www.doi.org/10.1016/j.jvs.2021.03.061
http://www.ncbi.nlm.nih.gov/pubmed/33940072?tool=bestpractice.com
Persistent type IA endoleak may necessitate conversion to open repair, provided the surgical risk is acceptable.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331.
https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2.
https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com
[207]Perini P, Bianchini Massoni C, Mariani E, et al. Systematic review and meta-analysis of the outcome of different treatments for type 1a endoleak after EVAR. Ann Vasc Surg. 2019 Oct;60:435-46.e1.
http://www.ncbi.nlm.nih.gov/pubmed/31200054?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Extension stent graft deployed for the same type I endoleak (encircled)University of Michigan/Dr G.R. Upchurch, Departments of Vascular Surgery and Radiology [Citation ends].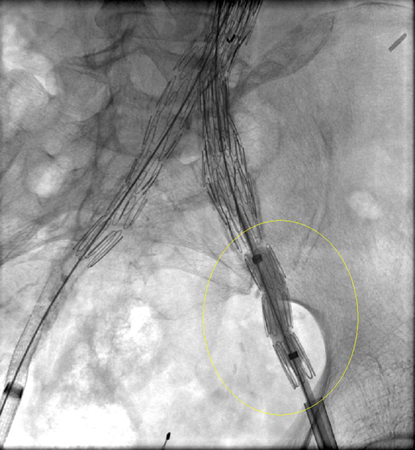 [Figure caption and citation for the preceding image starts]: Resolution of the type I endoleak resolved after extension deployedUniversity of Michigan/Dr G.R. Upchurch, Departments of Vascular Surgery and Radiology [Citation ends].
[Figure caption and citation for the preceding image starts]: Resolution of the type I endoleak resolved after extension deployedUniversity of Michigan/Dr G.R. Upchurch, Departments of Vascular Surgery and Radiology [Citation ends].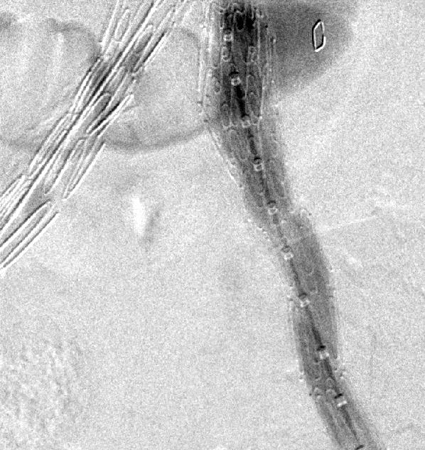
Type II: patent branch leak.[Figure caption and citation for the preceding image starts]: Type II endoleak (encircled) discovered on follow-up computed tomographyUniversity of Michigan/Dr G.R. Upchurch, Departments of Vascular Surgery and Radiology [Citation ends].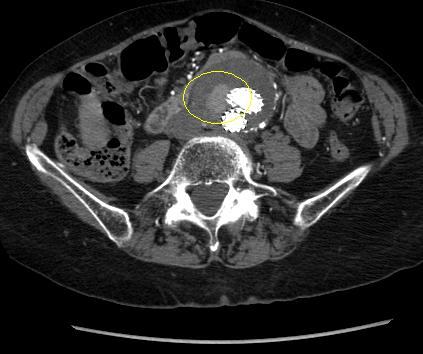 Spontaneous resolution may occur, although persistence may result in sac growth.[208]Higashiura W, Greenberg RK, Katz E, et al. Predictive factors, morphologic effects, and proposed treatment paradigm for type II endoleaks after repair of infrarenal abdominal aortic aneurysms. J Vasc Interv Radiol. 2007 Aug;18(8):975-81.
http://www.ncbi.nlm.nih.gov/pubmed/17675614?tool=bestpractice.com
If a type II endoleak or other abnormality of concern is observed on contrast-enhanced CT imaging at 1 month after EVAR, postoperative imaging at 6 months is recommended.[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2.
https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com
Around 50% of type II endoleaks are diagnosed before 30 day follow-up; 40% after 30 days, and 8% are diagnosed after 12 months of follow-up.[209]Charisis N, Bouris V, Conway AM, et al. A systematic review and pooled meta-analysis on the incidence and temporal occurrence of type II endoleak following an abdominal aortic aneurysm repair. Ann Vasc Surg. 2021 Aug;75:406-19.
http://www.ncbi.nlm.nih.gov/pubmed/33549794?tool=bestpractice.com
Treatment remains controversial and is advocated either if persistent at 6-12 months or when aneurysm sac size increases such that proximal and/or distal sealing zones may be compromised.[210]Mansueto G, Cenzi D, Scuro A, et al. Treatment of type II endoleak with a transcatheter transcaval approach: results at 1-year follow-up. J Vasc Surg. 2007 Jun;45(6):1120-7.
http://www.ncbi.nlm.nih.gov/pubmed/17543674?tool=bestpractice.com
[211]Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003 Sep;14(9 Pt 1):1111-7.
http://www.ncbi.nlm.nih.gov/pubmed/14514802?tool=bestpractice.com
[212]Van Marrewijk CJ, Fransen G, Laheij RJ, et al. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004 Feb;27(2):128-37.
http://www.ncbi.nlm.nih.gov/pubmed/14718893?tool=bestpractice.com
[213]Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg. 2000 Oct;32(4):739-49.
http://www.ncbi.nlm.nih.gov/pubmed/11013038?tool=bestpractice.com
[214]Ultee KHJ, Büttner S, Huurman R, et al. Editor's choice - systematic review and meta-analysis of the outcome of treatment for type II endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2018 Dec;56(6):794-807.
https://www.doi.org/10.1016/j.ejvs.2018.06.009
http://www.ncbi.nlm.nih.gov/pubmed/30104089?tool=bestpractice.com
[215]Smith T, Quencer KB. Best practice guidelines: imaging surveillance after endovascular aneurysm repair. AJR Am J Roentgenol. 2020 May;214(5):1165-74.
https://www.doi.org/10.2214/AJR.19.22197
http://www.ncbi.nlm.nih.gov/pubmed/32130043?tool=bestpractice.com
Treatment of choice is transarterial coil embolization, although laparoscopic ligation of collateral branches, direct percutaneous translumbar puncture of the sac, translumbar embolization, and transcatheter transcaval embolization have been reported.[205]Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002 May;35(5):1029-35.
http://www.ncbi.nlm.nih.gov/pubmed/12021724?tool=bestpractice.com
[210]Mansueto G, Cenzi D, Scuro A, et al. Treatment of type II endoleak with a transcatheter transcaval approach: results at 1-year follow-up. J Vasc Surg. 2007 Jun;45(6):1120-7.
http://www.ncbi.nlm.nih.gov/pubmed/17543674?tool=bestpractice.com
[211]Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003 Sep;14(9 Pt 1):1111-7.
http://www.ncbi.nlm.nih.gov/pubmed/14514802?tool=bestpractice.com
[212]Van Marrewijk CJ, Fransen G, Laheij RJ, et al. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004 Feb;27(2):128-37.
http://www.ncbi.nlm.nih.gov/pubmed/14718893?tool=bestpractice.com
[216]Steinmetz E, Rubin BG, Sanchez LA, et al. Type II endoleak after endovascular abdominal aortic aneurysm repair: a conservative approach with selective intervention is safe and cost-effective. J Vasc Surg. 2004 Feb;39(2):306-13.
http://www.ncbi.nlm.nih.gov/pubmed/14743129?tool=bestpractice.com
[217]Baum RA, Cope C, Fairman, et al. Translumbar embolization of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2001 Jan;12(1):111-6.
http://www.ncbi.nlm.nih.gov/pubmed/11200344?tool=bestpractice.com
[218]Baum RA, Carpenter JP, Golden MA, et al. Treatment of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms: comparison of transarterial and translumbar techniques. J Vasc Surg. 2002 Jan;35(1):23-9.
http://www.ncbi.nlm.nih.gov/pubmed/11802129?tool=bestpractice.com
[219]Schmid R, Gurke L, Aschwanden M, et al. CT-guided percutaneous embolization of a lumbar artery maintaining a type II endoleak. J Endovasc Ther. 2002 Apr;9(2):198-202.
http://www.ncbi.nlm.nih.gov/pubmed/12010100?tool=bestpractice.com
[220]Chin KM, Lee SQW, Lee HJ, et al. Preservation of stent graft after iatrogenic type III endoleak during open transperitoneal surgical intervention for complicated type II endoleak. Ann Vasc Surg. 2020 Jan;62:496.e1-496.e7.
http://www.ncbi.nlm.nih.gov/pubmed/31394250?tool=bestpractice.com
[221]Li Q, Hou P. Sac embolization and side branch embolization for preventing type II endoleaks after endovascular aneurysm repair: a meta-analysis. J Endovasc Ther. 2020 Feb;27(1):109-16.
https://www.doi.org/10.1177/1526602819878411
http://www.ncbi.nlm.nih.gov/pubmed/31566053?tool=bestpractice.com
[222]Akmal MM, Pabittei DR, Prapassaro T, et al. A systematic review of the current status of interventions for type II endoleak after EVAR for abdominal aortic aneurysms. Int J Surg. 2021 Nov;95:106138.
https://www.doi.org/10.1016/j.ijsu.2021.106138
http://www.ncbi.nlm.nih.gov/pubmed/34637951?tool=bestpractice.com
[223]Yu HYH, Lindström D, Wanhainen A, et al. Systematic review and meta-analysis of prophylactic aortic side branch embolization to prevent type II endoleaks. J Vasc Surg. 2020 Nov;72(5):1783-1792.e1.
https://www.doi.org/10.1016/j.jvs.2020.05.020
http://www.ncbi.nlm.nih.gov/pubmed/32442608?tool=bestpractice.com
[224]Zhang X, Ji L, Chen MY, et al. Transarterial embolization versus translumber embolization for type Ⅱ endoleak after endovascular abdominal aortic aneurysm repair: a meta-analysis. Chin Med Sci J. 2020 Jun 30;35(2):135-41.
https://www.doi.org/10.24920/003618
http://www.ncbi.nlm.nih.gov/pubmed/32684233?tool=bestpractice.com
Spontaneous resolution may occur, although persistence may result in sac growth.[208]Higashiura W, Greenberg RK, Katz E, et al. Predictive factors, morphologic effects, and proposed treatment paradigm for type II endoleaks after repair of infrarenal abdominal aortic aneurysms. J Vasc Interv Radiol. 2007 Aug;18(8):975-81.
http://www.ncbi.nlm.nih.gov/pubmed/17675614?tool=bestpractice.com
If a type II endoleak or other abnormality of concern is observed on contrast-enhanced CT imaging at 1 month after EVAR, postoperative imaging at 6 months is recommended.[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2.
https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com
Around 50% of type II endoleaks are diagnosed before 30 day follow-up; 40% after 30 days, and 8% are diagnosed after 12 months of follow-up.[209]Charisis N, Bouris V, Conway AM, et al. A systematic review and pooled meta-analysis on the incidence and temporal occurrence of type II endoleak following an abdominal aortic aneurysm repair. Ann Vasc Surg. 2021 Aug;75:406-19.
http://www.ncbi.nlm.nih.gov/pubmed/33549794?tool=bestpractice.com
Treatment remains controversial and is advocated either if persistent at 6-12 months or when aneurysm sac size increases such that proximal and/or distal sealing zones may be compromised.[210]Mansueto G, Cenzi D, Scuro A, et al. Treatment of type II endoleak with a transcatheter transcaval approach: results at 1-year follow-up. J Vasc Surg. 2007 Jun;45(6):1120-7.
http://www.ncbi.nlm.nih.gov/pubmed/17543674?tool=bestpractice.com
[211]Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003 Sep;14(9 Pt 1):1111-7.
http://www.ncbi.nlm.nih.gov/pubmed/14514802?tool=bestpractice.com
[212]Van Marrewijk CJ, Fransen G, Laheij RJ, et al. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004 Feb;27(2):128-37.
http://www.ncbi.nlm.nih.gov/pubmed/14718893?tool=bestpractice.com
[213]Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg. 2000 Oct;32(4):739-49.
http://www.ncbi.nlm.nih.gov/pubmed/11013038?tool=bestpractice.com
[214]Ultee KHJ, Büttner S, Huurman R, et al. Editor's choice - systematic review and meta-analysis of the outcome of treatment for type II endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2018 Dec;56(6):794-807.
https://www.doi.org/10.1016/j.ejvs.2018.06.009
http://www.ncbi.nlm.nih.gov/pubmed/30104089?tool=bestpractice.com
[215]Smith T, Quencer KB. Best practice guidelines: imaging surveillance after endovascular aneurysm repair. AJR Am J Roentgenol. 2020 May;214(5):1165-74.
https://www.doi.org/10.2214/AJR.19.22197
http://www.ncbi.nlm.nih.gov/pubmed/32130043?tool=bestpractice.com
Treatment of choice is transarterial coil embolization, although laparoscopic ligation of collateral branches, direct percutaneous translumbar puncture of the sac, translumbar embolization, and transcatheter transcaval embolization have been reported.[205]Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002 May;35(5):1029-35.
http://www.ncbi.nlm.nih.gov/pubmed/12021724?tool=bestpractice.com
[210]Mansueto G, Cenzi D, Scuro A, et al. Treatment of type II endoleak with a transcatheter transcaval approach: results at 1-year follow-up. J Vasc Surg. 2007 Jun;45(6):1120-7.
http://www.ncbi.nlm.nih.gov/pubmed/17543674?tool=bestpractice.com
[211]Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003 Sep;14(9 Pt 1):1111-7.
http://www.ncbi.nlm.nih.gov/pubmed/14514802?tool=bestpractice.com
[212]Van Marrewijk CJ, Fransen G, Laheij RJ, et al. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004 Feb;27(2):128-37.
http://www.ncbi.nlm.nih.gov/pubmed/14718893?tool=bestpractice.com
[216]Steinmetz E, Rubin BG, Sanchez LA, et al. Type II endoleak after endovascular abdominal aortic aneurysm repair: a conservative approach with selective intervention is safe and cost-effective. J Vasc Surg. 2004 Feb;39(2):306-13.
http://www.ncbi.nlm.nih.gov/pubmed/14743129?tool=bestpractice.com
[217]Baum RA, Cope C, Fairman, et al. Translumbar embolization of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2001 Jan;12(1):111-6.
http://www.ncbi.nlm.nih.gov/pubmed/11200344?tool=bestpractice.com
[218]Baum RA, Carpenter JP, Golden MA, et al. Treatment of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms: comparison of transarterial and translumbar techniques. J Vasc Surg. 2002 Jan;35(1):23-9.
http://www.ncbi.nlm.nih.gov/pubmed/11802129?tool=bestpractice.com
[219]Schmid R, Gurke L, Aschwanden M, et al. CT-guided percutaneous embolization of a lumbar artery maintaining a type II endoleak. J Endovasc Ther. 2002 Apr;9(2):198-202.
http://www.ncbi.nlm.nih.gov/pubmed/12010100?tool=bestpractice.com
[220]Chin KM, Lee SQW, Lee HJ, et al. Preservation of stent graft after iatrogenic type III endoleak during open transperitoneal surgical intervention for complicated type II endoleak. Ann Vasc Surg. 2020 Jan;62:496.e1-496.e7.
http://www.ncbi.nlm.nih.gov/pubmed/31394250?tool=bestpractice.com
[221]Li Q, Hou P. Sac embolization and side branch embolization for preventing type II endoleaks after endovascular aneurysm repair: a meta-analysis. J Endovasc Ther. 2020 Feb;27(1):109-16.
https://www.doi.org/10.1177/1526602819878411
http://www.ncbi.nlm.nih.gov/pubmed/31566053?tool=bestpractice.com
[222]Akmal MM, Pabittei DR, Prapassaro T, et al. A systematic review of the current status of interventions for type II endoleak after EVAR for abdominal aortic aneurysms. Int J Surg. 2021 Nov;95:106138.
https://www.doi.org/10.1016/j.ijsu.2021.106138
http://www.ncbi.nlm.nih.gov/pubmed/34637951?tool=bestpractice.com
[223]Yu HYH, Lindström D, Wanhainen A, et al. Systematic review and meta-analysis of prophylactic aortic side branch embolization to prevent type II endoleaks. J Vasc Surg. 2020 Nov;72(5):1783-1792.e1.
https://www.doi.org/10.1016/j.jvs.2020.05.020
http://www.ncbi.nlm.nih.gov/pubmed/32442608?tool=bestpractice.com
[224]Zhang X, Ji L, Chen MY, et al. Transarterial embolization versus translumber embolization for type Ⅱ endoleak after endovascular abdominal aortic aneurysm repair: a meta-analysis. Chin Med Sci J. 2020 Jun 30;35(2):135-41.
https://www.doi.org/10.24920/003618
http://www.ncbi.nlm.nih.gov/pubmed/32684233?tool=bestpractice.com
Type III: graft defect with leak through fabric tears, graft disconnection, or disintegration of the fabric.[204]Schurink GW, Aarts NJ, vanBockel JH. Endoleak after stent-graft treatment of abdominal aortic aneurysm: a meta-analysis of clinical studies. Br J Surg. 1999 May;86(5):581-7. http://www.ncbi.nlm.nih.gov/pubmed/10361173?tool=bestpractice.com [205]Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002 May;35(5):1029-35. http://www.ncbi.nlm.nih.gov/pubmed/12021724?tool=bestpractice.com [225]Kwon J, Dimuzio P, Salvatore D, et al. Incidence of stent graft failure from type IIIB endoleak in contemporary endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2020 Feb;71(2):645-53. https://www.doi.org/10.1016/j.jvs.2019.06.183 http://www.ncbi.nlm.nih.gov/pubmed/31466740?tool=bestpractice.com Repair is indicated upon discovery (endovascular stent graft extension).[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com [211]Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003 Sep;14(9 Pt 1):1111-7. http://www.ncbi.nlm.nih.gov/pubmed/14514802?tool=bestpractice.com [215]Smith T, Quencer KB. Best practice guidelines: imaging surveillance after endovascular aneurysm repair. AJR Am J Roentgenol. 2020 May;214(5):1165-74. https://www.doi.org/10.2214/AJR.19.22197 http://www.ncbi.nlm.nih.gov/pubmed/32130043?tool=bestpractice.com [226]Lowe C, Hansrani V, Madan M, et al. Type IIIb endoleak after elective endovascular aneurysm repair: a systematic review. J Cardiovasc Surg (Torino). 2020 Jun;61(3):308-16. http://www.ncbi.nlm.nih.gov/pubmed/29616524?tool=bestpractice.com
Type IV: leak from graft wall porosity.[204]Schurink GW, Aarts NJ, vanBockel JH. Endoleak after stent-graft treatment of abdominal aortic aneurysm: a meta-analysis of clinical studies. Br J Surg. 1999 May;86(5):581-7. http://www.ncbi.nlm.nih.gov/pubmed/10361173?tool=bestpractice.com [205]Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002 May;35(5):1029-35. http://www.ncbi.nlm.nih.gov/pubmed/12021724?tool=bestpractice.com These leaks are uncommon with newer stent grafts and are self-limited.[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com [211]Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003 Sep;14(9 Pt 1):1111-7. http://www.ncbi.nlm.nih.gov/pubmed/14514802?tool=bestpractice.com
Type V (endotension): endotension is increased intrasac pressure after EVAR without visualized endoleak on delayed contrast CT scans. less common with the newer-generation grafts.[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com There is no standardized method to measure endotension or consensus on indicated therapy in the absence of aneurysm enlargement; however, treatment of endotension to prevent aneurysm rupture is suggested in selected patients with continued aneurysm expansion.[76]Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. https://www.jvascsurg.org/article/S0741-5214(17)32369-8/fulltext http://www.ncbi.nlm.nih.gov/pubmed/29268916?tool=bestpractice.com [205]Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002 May;35(5):1029-35. http://www.ncbi.nlm.nih.gov/pubmed/12021724?tool=bestpractice.com [215]Smith T, Quencer KB. Best practice guidelines: imaging surveillance after endovascular aneurysm repair. AJR Am J Roentgenol. 2020 May;214(5):1165-74. https://www.doi.org/10.2214/AJR.19.22197 http://www.ncbi.nlm.nih.gov/pubmed/32130043?tool=bestpractice.com
preoperative cardiovascular risk reduction
Treatment recommended for ALL patients in selected patient group
Addressing modifiable cardiovascular risk factors preoperatively improves long-term survival after AAA repair.[194]Khashram M, Williman JA, Hider PN, et al. Management of modifiable vascular risk factors improves late survival following abdominal aortic aneurysm repair: a systematic review and meta-analysis. Ann Vasc Surg. 2017 Feb;39:301-11. http://www.ncbi.nlm.nih.gov/pubmed/27666804?tool=bestpractice.com
Patients should be encouraged to stop smoking and offered drug therapy (nicotine-replacement therapy, nortriptyline, and bupropion) or counseling to assist with this if needed.[1]Dehlin JM, Upchurch GR. Management of abdominal aortic aneurysms. Curr Treat Options Cardiovasc Med. 2005 Jun;7(2):119-30.
http://www.ncbi.nlm.nih.gov/pubmed/15935120?tool=bestpractice.com
[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482.
https://www.doi.org/10.1161/CIR.0000000000001106
http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
[13]Zankl AR, Schumacher H, Krumsdorf U, et al. Pathology, natural history and treatment of abdominal aortic aneurysms. Clin Res Cardiol. 2007 Mar;96(3):140-51.
http://www.ncbi.nlm.nih.gov/pubmed/17180573?tool=bestpractice.com
[15]Singh K, Bønaa H, Jacobsen BK, et al. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001 Aug 1;154(3):236-44.
https://academic.oup.com/aje/article/154/3/236/125840
http://www.ncbi.nlm.nih.gov/pubmed/11479188?tool=bestpractice.com
[22]Lederle FA, Johnson GR, Wilson SE, et al; Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Intern Med. 1997 Mar 15;126(6):441-9.
http://www.ncbi.nlm.nih.gov/pubmed/9072929?tool=bestpractice.com
[23]Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999 Dec;30(6):1099-105.
http://www.ncbi.nlm.nih.gov/pubmed/10587395?tool=bestpractice.com
[134]Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018 May 31;5:CD000146.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000146.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/29852054?tool=bestpractice.com
[135]Rigotti NA, Clair C, Munafò MR, et al. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012 May 16;(5):CD001837.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001837.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/22592676?tool=bestpractice.com
[136]Howes S, Hartmann-Boyce J, Livingstone-Banks J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2020 Apr 22;(4):CD000031.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000031.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/32319681?tool=bestpractice.com
[  ]
What are the effects of adding bupropion or fluoxetine to other treatments compared with using other treatments alone for people trying to quit smoking?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4337/fullShow me the answer
]
What are the effects of adding bupropion or fluoxetine to other treatments compared with using other treatments alone for people trying to quit smoking?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4337/fullShow me the answer
Preoperative exercise training reduced postsurgical cardiac complications in a small randomized controlled trial (RCT) of patients undergoing open or endovascular AAA repair, though a Cochrane review and a separate systematic review of prehabilitation (exercise training) prior to AAA surgery did not show any outcome benefit.[195]Barakat HM, Shahin Y, Khan JA, et al. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair: a randomized controlled trial. Ann Surg. 2016 Jul;264(1):47-53. http://www.ncbi.nlm.nih.gov/pubmed/26756766?tool=bestpractice.com [196]Fenton C, Tan AR, Abaraogu UO, et al. Prehabilitation exercise therapy before elective abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2021 Jul 8;7(7):CD013662. https://www.doi.org/10.1002/14651858.CD013662.pub2 http://www.ncbi.nlm.nih.gov/pubmed/34236703?tool=bestpractice.com [197]Bonner RJ, Wallace T, Jones AD, et al. The content of pre-habilitative interventions for patients undergoing repair of abdominal aortic aneurysms and their effect on post-operative outcomes: a systematic review. Eur J Vasc Endovasc Surg. 2021 May;61(5):756-65. https://www.doi.org/10.1016/j.ejvs.2021.01.043 http://www.ncbi.nlm.nih.gov/pubmed/33678532?tool=bestpractice.com While preoperative exercise training may be beneficial for patients undergoing AAA repair, further investigation with RCTs is needed before it can be recommended more widely.[198]Wee IJY, Choong AMTL. A systematic review of the impact of preoperative exercise for patients with abdominal aortic aneurysm. J Vasc Surg. 2020 Jun;71(6):2123-31.e1. https://www.doi.org/10.1016/j.jvs.2018.09.039 http://www.ncbi.nlm.nih.gov/pubmed/30606665?tool=bestpractice.com
Perioperative statin use slows aneurysm growth, reduces risk of rupture and, reduces mortality from AAA repair or ruptured AAA.[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com Statins should be started at least 1 month before surgery to reduce cardiovascular morbidity and mortality, and continued indefinitely.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [139]Risum Ø, Sandven I, Sundhagen JO, et al. Editor's choice - effect of statins on total mortality in abdominal aortic aneurysm repair: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2021 Jan;61(1):114-20. https://www.doi.org/10.1016/j.ejvs.2020.08.007 http://www.ncbi.nlm.nih.gov/pubmed/32928667?tool=bestpractice.com
Patients with AAAs are at increased risk of major adverse cardiovascular events. There is limited evidence, but in the absence of any contraindication, patients with AAA should receive single antiplatelet therapy (aspirin or clopidogrel).[5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com [140]Aboyans V, Bauersachs R, Mazzolai L, et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: a consensus document from the ESC working group on aorta and peripheral vascular diseases, the ESC working group on thrombosis, and the ESC working group on cardiovascular pharmacotherapy. Eur Heart J. 2021 Oct 14;42(39):4013-24. https://www.doi.org/10.1093/eurheartj/ehab390 http://www.ncbi.nlm.nih.gov/pubmed/34279602?tool=bestpractice.com This should be continued during the perioperative period.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com
Hypertension should be controlled to reduce cardiovascular morbidity and mortality.[3]Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's choice -- European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024 Feb;67(2):192-331. https://www.ejves.com/article/S1078-5884(23)00889-4/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38307694?tool=bestpractice.com [5]Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Dec 13;146(24):e334-e482. https://www.doi.org/10.1161/CIR.0000000000001106 http://www.ncbi.nlm.nih.gov/pubmed/36322642?tool=bestpractice.com
perioperative antibiotic therapy
Treatment recommended for ALL patients in selected patient group
Perioperative antibiotic therapy is given. Broad-spectrum antibiotic coverage is necessary, in accordance with local protocols.

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer