Approach
Most arteriovenous malformations (AVMs) are diagnosed as part of a diagnostic workup for other conditions such as intracerebral hemorrhage (ICH) or seizure. Technological advances and increased availability of brain imaging have increased the rate of detection of AVMs, both symptomatic and incidental.
Clinical presentation
AVMs most commonly present with ICH (40% to 70%).[1][2][3] Symptoms and signs of ICH relate to either 1) focal neurologic deficits or seizures arising from injury to the brain parenchyma, or 2) symptoms of mass effect, elevated intracranial pressure from the hematoma such as severe headache, nausea and vomiting, confusion, drowsiness, and coma.
Unruptured AVMs are often found incidentally or may present with focal neurologic symptoms resulting from local mass effect, inflammation, or altered hemodynamics such as "vascular steal". Between 5% and 15% of AVMs present with progressive neurologic deficits unrelated to hemorrhage.[46] The most common sequela is epilepsy, which is present in about 20% to 30% of patients.[2][4][5]
Initial investigations
A brain computed tomography (CT) scan is an extremely useful initial study to confirm or exclude ICH. AVMs may also be visible as areas of mixed density with edema, mass effect, and enhancement with contrast. Calcification is seen in 25% to 30% of AVMs.[47][48] If there is evidence of hematoma, the pattern of hematoma in the context of the patient's age and past medical history influences the differential diagnosis and further management. [Figure caption and citation for the preceding image starts]: Left posterior frontal intracerebral hematoma secondary to ruptured arteriovenous malformation (axial unenhanced computed tomography scan)From the collection of Mr R. J. Edwards; used with permission [Citation ends].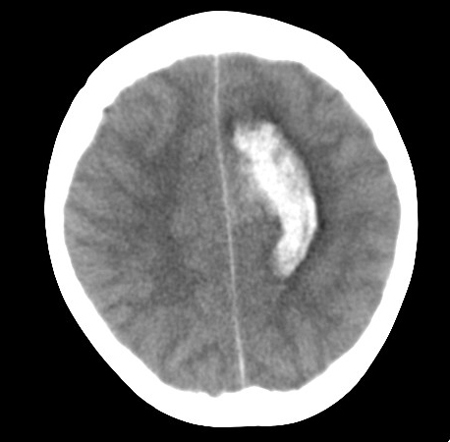
Further evaluation with magnetic resonance imaging (MRI) should be sought in the event of a negative CT scan and other symptoms compatible with AVM (e.g., seizure). MRI has a sensitivity of 89% for detecting AVMs and offers greater visualization of surrounding structures than CT.[45][49] The AVM vessels appear as "flow voids" if they contain high-velocity blood or as a high signal if they are thrombosed or contain turbulent blood flow within areas of gliosis (hyperintense on T2). The signal characteristics of any hemorrhage depend on its age. The use of gadolinium enhancement and sequences that detect blood products increases the sensitivity for small AVMs.[Figure caption and citation for the preceding image starts]: Unruptured left parieto-occipital arteriovenous malformation (sagittal T1-weighted magnetic resonance imaging scan)From the collection of Mr R. J. Edwards; used with permission [Citation ends].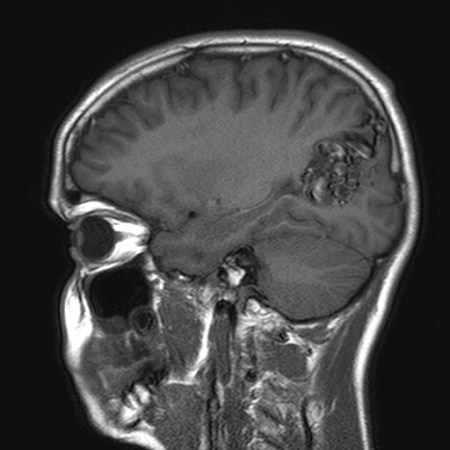 [Figure caption and citation for the preceding image starts]: Unruptured left parieto-occipital arteriovenous malformation (axial T2-weighted magnetic resonance imaging scan)From the collection of Mr R. J. Edwards; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Unruptured left parieto-occipital arteriovenous malformation (axial T2-weighted magnetic resonance imaging scan)From the collection of Mr R. J. Edwards; used with permission [Citation ends].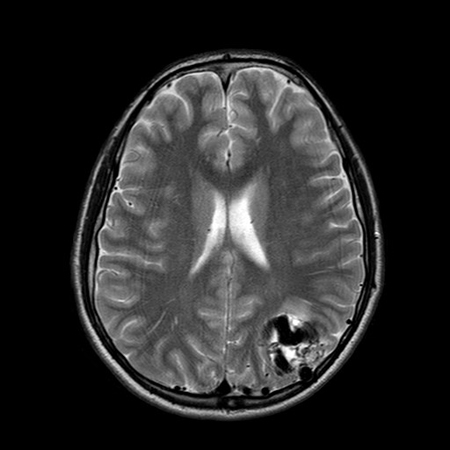
Subsequent investigations
Digital subtraction angiography (DSA)
This is the standard investigation that provides the highest spatial resolution for determining AVM size, location, feeding arteries, associated aneurysms, and venous drainage.
The characteristic diagnostic feature is early venous filling (indicating arteriovenous [AV] shunting) during the arterial phase. However, this may also be seen with vascular neoplastic lesions, contusions, parenchymal infection, and following infarcts.[47] The AVM appears as a tangle of tightly packed abnormal vessels with enlarged feeding arteries and dilated, tortuous draining veins. They are often wedge shaped, pointing toward the ventricle. [Figure caption and citation for the preceding image starts]: Cerebral angiogram (left carotid artery injection, lateral view) showing posterior frontal arteriovenous malformation fed by pericallosal artery (thin arrow) with arterialized draining vein (thick arrow) draining to superior sagittal sinusFrom the collection of Mr R. J. Edwards; used with permission [Citation ends].
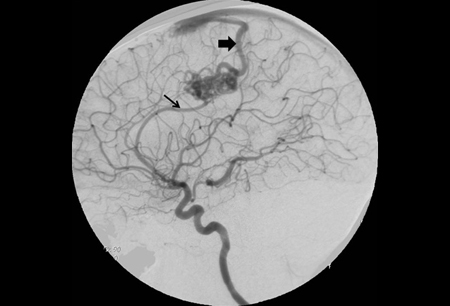
Microarteriovenous malformations are AVMs with a nidus diameter of <1 cm. They account for 7% of all cerebral AVMs, and 21% of those presenting with ICH.[50] They are typically the source of large intracerebral hematomas in young adults. On careful analysis of angiography, most microarteriovenous malformations can be detected by the presence of subtle features such as capillary blush or early venous filling (indicating AV shunting) during the arterial phase. However, some are angiographically occult.
DSA investigation of ICH is warranted in patients <60 years of age or with a bleeding pattern that is not characteristic of other causes, such as hypertension or amyloid angiopathy, and if the patient is medically fit enough to consider further treatment.[51]
CT and magnetic resonance angiograms
High-definition angiographic-type images can be obtained from CT and magnetic resonance angiograms without the risks of formal angiography. The technique involves giving intravenous contrast agent at the time of the scan. The images are captured and processed to display the cerebral vasculature.
They can identify most AVMs but lack sensitivity to detect small AV shunts. CT and magnetic resonance angiograms can be extremely useful adjuncts to the investigation of a suspected AVM and may be used in follow-up after treatment.
Functional imaging
Standard imaging techniques such as positron emission tomography (PET), functional MRI, or magnetoencephalography provide accurate anatomic localization of eloquent areas (areas of the brain that control speech, motor function, and senses).
Functional imaging identifies functionally important cortex that may not correspond with that which would be expected anatomically, as neuronal networks seem to adapt to the presence of AVMs.[52][53]
Superselective Wada testing
The classic Wada test of injecting sodium amobarbital into the internal carotid artery is used before epilepsy surgery to establish the lateralization of language and memory.
Endovascular embolization of the arterial supply to AVMs is commonly used as an adjunct to surgery or radiosurgery. Before the deployment of the sclerosant, it is becoming increasingly common to assess the potential consequences by injecting sodium amobarbital.
This test has revealed the redistribution of cognitive function in response to AVMs.[54] It is a means of assessing the function of the surrounding cortex before surgery, although functional MRI is more commonly used as it is noninvasive and has fewer complications and adverse effects.
PET
PET is the most accurate method to evaluate brain hemodynamics and can also be used to provide functional information.
Laboratory studies
Complete blood count, clotting screen, blood group, and renal function tests should be performed in patients presenting with hemorrhage or in any patient before surgery, in order to exclude coagulopathies and to ensure that blood can be readily cross-matched.
A drug toxicology screen is recommended in young adults presenting with hemorrhage, as illicit drug use is the most common risk factor for stroke in this patient population.
Electroencephalogram
This is indicated in patients presenting with seizures.
If the location of the seizure focus is in doubt and surgical resection is being considered, more detailed neurophysiology, such as video telemetry, and more invasive (e.g., subdural grid) electrode monitoring can be used.
Neuropsychology
In the presence of AVMs, neurocognitive function may be in an unusual cerebral location, some distance from any effects of "vascular steal".[54] Eloquent cortical regions may not occupy their normal anatomic location; therefore, establishing an individual cortical functional map using neuropsychology and functional imaging before surgery is useful.
Visual fields testing
This test is appropriate if an AVM or hemorrhage encroaches on visual pathways.
Use of this content is subject to our disclaimer