Etiology
Congenital
Ventricular septal defects (VSDs) are usually developmental congenital conditions. No definite cause is known. In families with a strong history of congenital cardiovascular malformations, the incidence of VSD is increased. Certain genetic abnormalities have a high incidence of associated VSD, the most common being Down syndrome, which is associated with congenital cardiovascular malformations in one third to one half of cases.[14][15]
Acquired
In rare cases, a VSD can occur as a result of acute myocardial infarction (MI). This usually occurs 2 to 5 days after the infarction and is marked by the onset of acute left-heart failure, chest pain, low cardiac output and shock.[6] VSD also occurs as a result of penetrating or, rarely, blunt trauma.
Pathophysiology
The pathophysiologies of all VSDs are essentially the same, regardless of location. Two factors determine the hemodynamic effects of a VSD: the size of the defect and the pulmonary vascular resistance. If the defect is able to limit the transmission of pressure from the left to the right side of the heart, it is called a restrictive defect.
A small restrictive defect (pulmonary to systemic blood flow ratio [Qp:Qs] <1.5:1) leads to minimal left-to-right shunting across the ventricles, and is not associated with any increase in pulmonary vascular resistance or with any symptoms. If found in infancy there is a high likelihood of spontaneous closure; however, it has a good prognosis, even if it persists into adulthood.
A moderate restrictive defect (Qp:Qs 1.5:1 to 2.5:1) will lead to left-to-right shunting and a variable amount of pulmonary hypertension. Initially, there is reactive pulmonary hypertension (pulmonary hypertension that is reversible with pulmonary vasodilators) with left-to-right shunting. Gradually, the pulmonary vascular resistance rises, resulting in decreased shunting. Persistent pulmonary hypertension eventually becomes "fixed" pulmonary hypertension, produced by permanent irreversible remodeling of the pulmonary vasculature. Pulmonary hypertension increases the back pressure on the heart, causing dilatation of the cardiac chambers and, eventually, heart failure.
In larger defects (Qp:Qs >2.5:1) and in late stages, significant pulmonary hypertension occurs and pulmonary vascular resistance rises to the point that the shunt reverses (Eisenmenger syndrome). Shunt reversal alludes to blood flowing from the right to the left ventricle, effectively "reversing" the flow seen through most VSDs. It results in unoxygenated blood from the systemic venous return passing directly into the systemic arterial circulation without going through the lungs to be oxygenated. Oxygen saturation in the systemic arterial circulation therefore falls, resulting in cyanosis. The VSD becomes inoperable at this point.
In infancy, the mechanism of pulmonary hypertension is different from in adults, and is primarily due to increased pulmonary blood flow rather than increased pulmonary vascular resistance. However, the sequelae are the same. Reduced systemic perfusion is still seen in infants, resulting in heart failure.
An acute VSD resulting from an acute MI will usually result in acute left-heart failure with pulmonary edema and shock.[6]
Additional problems can arise with certain types of VSD. With type 1 defects, accompanying aortic regurgitation is common, produced by prolapse of the anterior aortic valve leaflet. With type 3 defects, there is often involvement of the atrioventricular valves and the atrial septum.
Classification
Classification according to location[1]
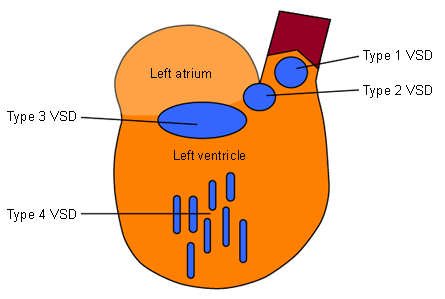
VSDs are classified according to their location. There are 4 main types, 3 of which are known by several synonyms.
Type 1
6% of isolated VSDs in non-Asian populations, but up to 33% in Asian populations
Synonyms: conal defect, conal septal hypoplasia, sub-pulmonary defect, infundibular defect, supracristal defect, doubly committed juxta-arterial defect
Location: lies beneath the semilunar valves in the conal or outlet septum
Often associated with aortic regurgitation produced by prolapse of the anterior aortic valve leaflet
Type 2
Synonyms: perimembranous defect, paramembranous defect, conoventricular defect
Location: confluent with the membranous septum, bordered by an atrioventricular (AV) valve
Type 3
Synonyms: peri-inlet defect, AV canal type defect, AV septal defect, endocardial cushion defect
Location: involves the inlet of the ventricular septum immediately inferior to the AV valve apparatus
Typically occur in patients with Down syndrome
Type 4
Up to 20% of VSDs in infants. Adult incidence is much lower
Synonym: muscular defect
Location: completely surrounded by muscle; multiple defects may be present, producing a "Swiss cheese" appearance of the septum
Spontaneous closure may occur[Figure caption and citation for the preceding image starts]: Simplified diagram of the left ventricular septum showing the anatomical locations of the ventricular septal defectsFrom the collection of Dr Zuhdi Lababidi [Citation ends].
Classification by etiology
Congenital
Most VSDs are the result of a developmental defect
Acquired
Rare
Necrosis of the septum following MI can produce a VSD
Penetrating trauma (e.g., a stab wound) or, very rarely, blunt trauma can produce a VSD
Classification by size[2]
VSDs can be divided into small, moderate, and large defects according to size. The magnitude of the shunt produced by a VSD is described by the Qp:Qs ratio.
The 2018 American Heart Association/American College of Cardiology guideline includes the following classification:
Small restrictive defects. The pulmonary vascular resistance is not significantly elevated and the left-to-right shunt is small (Qp:Qs <1.5:1).
Large nonrestrictive defects in cyanotic patients who have developed Eisenmenger syndrome, with pulmonary vascular resistance at systemic levels and shunt reversal (right-to-left).
Patients with moderately restrictive defects (Qp:Qs ≥1.5:1 and <2:1) who have not undergone closure for some reason. These patients often have mild-to-moderate pulmonary arterial hypertension.
Patients who have had their defects closed in childhood. These patients may have VSD patch leaks.
International Society for Nomenclature of Paediatric and Congenital Heart Disease classification scheme for VSD[3]
Alternate classification of VSD
Perimembranous central VSD
Inlet VSD without a common AV junction
Inlet perimembranous VSD without AV septal malalignment and without a common AV junction
Inlet perimembranous VSD with AV septal malalignment and without a common AV junction
Inlet muscular VSD
Trabecular muscular VSD
Trabecular muscular VSD: midseptal
Trabecular muscular VSD: apical
Trabecular muscular VSD: postero-inferior
Trabecular muscular VSD: anterosuperior
Trabecular muscular VSD: multiple (“Swiss cheese” septum)
Outlet VSD
Outlet VSD without malalignment
Outlet muscular VSD without malalignment
Doubly committed juxta-arterial VSD without malalignment
Doubly committed juxta-arterial VSD without malalignment and with a muscular postero-inferior rim
Doubly committed juxta-arterial VSD without malalignment and with a fibrous postero-inferior rim (perimembranous extension)
Outlet VSD with anteriorly malaligned outlet septum
Outlet muscular VSD with anteriorly malaligned outlet septum
Outlet perimembranous VSD with anteriorly malaligned outlet septum
Doubly committed juxta-arterial VSD with anteriorly malaligned fibrous outlet septum
Doubly committed juxta-arterial VSD with anteriorly malaligned fibrous outlet septum and a muscular postero-inferior rim
Doubly committed juxta-arterial VSD with anteriorly malaligned fibrous outlet septum and a fibrous postero-inferior rim (perimembranous extension)
Outlet VSD with posteriorly malaligned outlet septum
Outlet muscular VSD with posteriorly malaligned outlet septum
Outlet perimembranous VSD with posteriorly malaligned outlet septum
Doubly committed juxta-arterial VSD with posteriorly malaligned fibrous outlet septum
Doubly committed juxta-arterial VSD with posteriorly malaligned fibrous outlet septum and a muscular postero-inferior rim
Doubly committed juxta-arterial VSD with posteriorly malaligned fibrous outlet septum and a fibrous postero-inferior rim (perimembranous extension)
International Classification of Diseases, 11th revision (ICD-11)[4]
In ICD-11, VSDs are contained within the “Developmental anomalies” chapter and are defined as a congenital cardiac malformation in which there is a hole or pathway between the ventricular chambers. They are further subclassified as follows.
Trabecular muscular ventricular septal defect
A congenital cardiac malformation in which there is a ventricular septal defect within the trabeculated component of the ventricular septum.
Perimembranous central ventricular septal defect
A congenital cardiovascular malformation in which there is a ventricular septal defect that:
occupies the space that is usually closed by the interventricular part of the membranous septum
is usually adjacent to the area of fibrous continuity between the leaflets of an atrioventricular valve and an arterial valve
is adjacent to an area of mitral-tricuspid fibrous continuity
is located at the center of the base of the ventricular mass.
Ventricular septal defect hemodynamically insignificant
A congenital cardiac malformation in which there is one or more small, clinically insignificant ventricular septal defect(s) in the absence of flow-related cardiac chamber dilation or abnormal elevation of pulmonary arterial pressure.
Use of this content is subject to our disclaimer