Approach
Persistent cough with or without hemoptysis and weight loss in a smoker over the age of 50 are key features that should alert the clinician to the possibility of lung cancer. However, lung cancer can present without symptoms as an incidental mass on chest x-ray or computed tomography (CT).
History and symptoms
Smoking history, nutritional status, and performance status (an objective assessment of the patient's ability to perform activities of daily living) should be specifically addressed.
Symptoms of a primary tumor include cough, hemoptysis, chest pain, shoulder pain, and/or dyspnea. Some patients may present with hoarseness secondary to recurrent laryngeal nerve paralysis. Patients may also present with nonspecific symptoms such as weight loss or fatigue.[45]
Most patients develop distant metastasis during the course of their disease. The most frequent sites of distant metastasis are the liver, brain, bone, and adrenal glands. Symptoms depend on the sites and extent of involvement. Pain or fractures can develop as a result of bone metastasis. Lung cancer is the most common cause of brain metastasis.[46][47] Common symptoms include confusion, personality change, seizures, weakness, focal neurologic deficits, nausea and vomiting, and headaches.
Physical exam
The general appearance of the patient is important. The patient may appear ill and short of breath, and have evidence of recent weight loss. The neck and supraclavicular fossa should be carefully examined for lymphadenopathy (ultrasound of the neck may be used if available). Finger clubbing and hypertrophic osteoarthropathy may be present.
Although the pulmonary exam is often normal in patients with early lung cancer, many present with one or more findings during auscultation. The following physical signs are common: wheezing from underlying chronic obstructive pulmonary disease (COPD) or bronchial obstruction; rales due to postobstructive pneumonia or atelectasis; and diminished breath sounds from bronchial obstruction, pleural effusion, and/or COPD. Pleural effusions can be assessed with percussion of the lung fields, showing a characteristic dullness.
Facial and upper extremity swelling, distended neck veins, and dilated collateral vessels on the chest or abdominal wall may indicate compression of the superior vena cava.
Superior sulcus tumors can invade the brachial plexus, causing weakness and/or atrophy of the intrinsic muscles of the hand, and paresthesias and/or pain in a C8/T1 distribution. Additionally, these tumors can impact the sympathetic chain, causing Horner syndrome (ptosis, miosis, and ipsilateral anhidrosis).
Investigations
The initial approach to investigations is based on the clinical history, examination findings, and usually a baseline contrast-enhanced CT scan of the lower neck, thorax, and upper abdomen.
A diagnostic multidisciplinary team (MDT) should provide an opinion on the sequence of investigations that gives the most diagnostic, staging, and fitness information with the least risk. At a minimum, this team should include a pulmonologist, a diagnostic radiologist, and a coordinator. More complex cases may involve a wider team; for example, thoracic surgeon, radiation oncologist, medical oncologists, and pulmonary pathologists.
Initial imaging studies
A standard posteroanterior (PA) chest x-ray is a simple initial step to evaluate cough, chest pain, and/or hemoptysis. In some centers a lateral chest x-ray may be performed as well. For symptoms such as persistent hemoptysis, it is common practice to omit the chest x-ray and perform CT.
A new abnormality on chest x-ray needs to be further assessed with contrast-enhanced CT.[48] A chest CT should be obtained in patients, especially smokers, with problematic symptoms and a normal chest x-ray.
A contrast-enhanced CT of the lower neck, chest, and upper abdomen is standard and helps to define the primary tumor and evaluate for regional spread. Imaging should be directed at potential metastasis sites when symptoms or focal findings are present or when there is evidence of advanced disease on chest CT.
Pathologic confirmation
In some cases pathologic confirmation is only finally established when the lesion is surgically resected. Tissue sample depends on the location of the lesion. The aim should be to obtain adequate diagnostic and staging information to guide treatment with least harm and distress to the patient.[49][50][51][52]
Sputum cytology: specificity is high, but the sensitivity is low.[50] Cytology is more likely to confirm the diagnosis in central lesions than in peripheral lesions.[50] Sputum cytology cannot accurately determine histology or inform appropriate therapy.
Video-assisted thoracic surgery (VATS): a minimally invasive procedure that can be employed in the biopsy of most primary tumors.
Transbronchial needle aspiration biopsy: for the evaluation of accessible parenchymal lesions and mediastinal lymph nodes; can be carried out with or without endobronchial ultrasound (EBUS) guidance. The use of EBUS expands the number and levels of mediastinal nodes that can be sampled. Endoscopic ultrasound (EUS) can also be used to access other mediastinal glands plus the left adrenal gland but overall EBUS is a more useful primary procedure.[53]
Standard bronchoscopy: indicated where CT shows a lesion accessible via bronchoscopy and a biopsy will yield the necessary diagnostic and staging information. If staging is important (e.g., of mediastinal adenopathy), EBUS is performed instead. Bronchoscopy is also used to assess new and/or unexplained pulmonary symptoms (e.g., hemoptysis, wheezing, cough). Flexible bronchoscopy usually requires conscious sedation. During the procedure, the tracheobronchial tree is carefully examined. Endobronchial tumors can be biopsied. Washings, brushings, and bronchoalveolar lavage are performed. Suspicious parenchymal lesions and mediastinal lymph nodes that are accessible can also be biopsied. EBUS can also be used to biopsy mediastinal lymph nodes and some submucosal tumors, which can provide information regarding tissue diagnosis and stage in a single procedure.[54][55][56][57] Autofluorescent or narrow band imaging can improve the detection of early endobronchial lesions and can be used at the time of initial bronchoscopy or follow-up.[58][59] Image-guided bronchoscopy techniques such as electromagnetic navigational bronchoscopy may be used for some peripheral lesions.[60] Bronchoscopy is a safe procedure, with a mortality of <1 in 4000.
Transthoracic needle aspiration biopsy: often necessary for peripheral lesions that are inaccessible to bronchoscopy. It is indicated either where there is no need for detailed staging or where there are no lymph node or other potential metastases amenable to biopsy. Transthoracic biopsy has the complications of hemorrhage and pneumothorax.[61]
Diagnostic thoracentesis or pleural biopsy using thoracoscopy or CT-guided biopsy: for the evaluation of pleural effusions (relatively common in lung cancer patients, portending poor prognosis) if there is no other evidence of metastatic disease. Ultrasound guidance is preferred for all medical pleural procedures and is essential when sampling small pleural effusions.
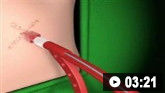
Insertion of intercostal drain, Seldinger technique: animated demonstrationHow to insert an intercostal (chest) drain using the Seldinger technique. Video demonstrates: how to identify a safe site for insertion; use of an introducer needle, guidewire, dilators, and intercostal drain; how to confirm drain position; and postprocedure care.
CT-guided biopsy of the pleura: preferred approach for pleural thickening shown on CT. Thoracoscopy (medical or surgical) is a highly reliable method of tissue confirmation of pleural effusions.
Staging
Where possible, diagnosis and staging is done in parallel so that the diagnostic biopsy also gives pivotal staging information that allows treatment to proceed.[66] Various investigations are useful in clarifying the stage, either before or after the first biopsy.
CT with contrast and/or fluorodeoxyglucose (FDG)-PET: can be considered to evaluate progression. FDG-PET/CT is performed from the skull base to mid-thigh. Positive FDG-PET/CT scan findings for distant disease need pathologic or other radiologic confirmation. If FDG-PET/CT scan is positive in the mediastinum, lymph node status needs pathologic confirmation.[67]
Tissue sampling of the mediastinum via mediastinoscopy or endobronchial ultrasound (EBUS): the most accurate method to stage the mediastinum, and is often indicated following FDG-PET/CT. European guidance recommends combined EBUS and EUS for mediastinal staging of lung cancer; it is less invasive than mediastinoscopy and may avoid unnecessary thoracotomy.[68][69]
Video-assisted thoracoscopic surgery (VATS) can be used to evaluate aorticopulmonary window lymph nodes. VATS also allows access to paraesophageal and pulmonary ligament lymph nodes.
Ultrasound-guided needle biopsy of supraclavicular lymph nodes: valuable in some patients even when no nodes are palpable.[70]
Bone scan: not routinely recommended; FDG PET/CT has higher sensitivity.[71] May be considered in the absence of FDG PET/CT in patients with new bone pain or elevated alkaline phosphatase.
CT or magnetic resonance imaging (MRI) head: indicated in patients with early-stage NSCLC if brain metastases are suspected based on symptoms. Unless contra-indicated (such as patients with certain metal prosthesis, etc.), MRI is preferred, as it is more sensitive. Patients with locally advanced NSCLC, especially adenocarcinoma, are at higher risk of harboring brain metastases and should be staged with a head CT or MRI if aggressive local therapy is recommended.
MRI of the thoracic inlet: may be helpful in assessing resectability of superior sulcus tumors, although high-quality multidetector CT imaging is probably as good in most circumstances.
Molecular biomarkers
Targeted therapy may be available for patients presenting with specific gene mutations or fusions (molecular biomarkers). A large next generation sequencing (NGS) panel can test for multiple biomarkers simultaneously.[67][72]
The following mutations or fusions are relevant to NSCLC.[67]
Epidermal growth factor receptor (EGFR) mutations: testing for mutations of the tyrosine kinase genes that encode EGFR in tumor cells enables targeted therapy to be considered in a subset of patients, particularly those who are ex-light smokers or never-smokers and those with nonsquamous histology (i.e., adenocarcinoma and large cell carcinoma). Patients with sensitizing EGFR-mutations preferentially benefit from EGFR tyrosine kinase inhibitor therapy over chemotherapy.
Anaplastic lymphoma kinase (ALK) gene rearrangements: testing for mutations of the tyrosine kinase genes that encode ALK in tumor cells enables targeted therapy to be considered in a subset of patients, particularly those who are ex-light smokers or never-smokers and those with nonsquamous histology (i.e., adenocarcinoma and large cell carcinoma). ALK-positive patients preferentially benefit from ALK-inhibitor therapy over chemotherapy.
ROS proto-oncogene 1 (ROS1) gene fusions: a receptor tyrosine kinase that may be rearranged in NSCLC. ROS1 fusions are identified in around 1% to 2% of patients and are usually detected by fluorescence in situ hybridization (FISH). Testing enables consideration of ROS1-directed therapy with tyrosine kinase inhibitors. ROS1 fusions are more prevalent in younger patients, never-smokers, those with a history of light smoking, and patients with adenocarcinomas.[73]
B-Raf proto-oncogene (BRAF) point mutations: presence of the BRAF V600E genotype (found in 1% to 2% of adenocarcinomas) assists decision-making for BRAF inhibitor-directed therapy.[74] One meta-analysis reported a significant association between BRAF mutations and adenocarcinoma in NSCLC patients; BRAF mutations were more frequent in women. The BRAF V600E mutation was significantly associated with NSCLC in women and nonsmokers.[75]
Neurotrophin tyrosine receptor kinase (NTRK) gene fusions: tropomyosin receptor kinase (TRK) inhibitors are recommended for NTRK fusions.
Mesenchymal-epithelial transition factor (MET) exon 14 (METex14) skipping mutations: c-MET inhibitors are recommended.
Rearranged during transfection (RET) gene mutations: the RET gene is a proto-oncogene that may be mutated in patients with NSCLC. RET rearrangements are more common in patients with adenocarcinoma.[76] In European patients, RET rearrangements can occur in both smokers and nonsmokers.[77] RET kinase inhibitors are recommended.
KRAS proto-oncogene (KRAS) point mutations: inhibitors of the RAS GTPase family are indicated for the treatment of KRAS G12C-mutated NSCLC.
Erb-B2 receptor tyrosine kinase 2 (ERBB2 [HER2]) gene mutations: ERBB2 encodes for HER2, a receptor tyrosine kinase. Fam-trastuzumab deruxtecan is recommended as subsequent therapy.
Immune biomarkers
Testing for immune biomarkers may identify patients eligible for immunotherapy.
Programmed death-ligand 1 (PD-L1) expression
Testing for PD-L1 expression in patients with metastatic NSCLC may identify candidates for PD-L1 inhibitor therapy.[67] PD-L1 status can be evaluated on cytologic samples or biopsies; the SP142 assay has lower sensitivity than the 22C3, 28-8, and SP263 assays, which performed similarly in evaluating PD-L1 expression quantity on tumor cells.[78][79]
Ancillary studies
Baseline tests before initiation of treatment include:
Complete blood count
Clotting studies
Chemistry panel
Liver function tests, including alkaline phosphatase
Testing may detect paraneoplastic syndromes such as hypercalcemia of malignancy, cancer-related anemia, hyponatremia secondary to inappropriate antidiuretic hormone (ADH) secretion, and, rarely, ectopic adrenocorticotropic hormone (ACTH) secretion (Cushing syndrome, resulting in hyperglycemia and hypokalemia). In metastatic disease, alkaline phosphatase levels can be elevated, indicating potential bone or liver metastases.
All lung cancer patients anticipated to receive chest radiation therapy or surgery should have pulmonary function tests (PFTs) performed, including FEV₁ and diffusing capacity of the lung for carbon monoxide (the latter where spirometry is abnormal or dyspnea is present).[80][81]
Electrocardiogram (ECG) is always performed preoperatively. Echocardiogram may be done if there is pre-existing cardiac disease or unexpectedly low exercise tolerance.
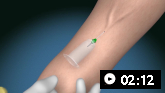
How to take a venous blood sample from the antecubital fossa using a vacuum needle.
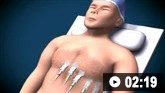
How to record an ECG. Demonstrates placement of chest and limb electrodes.
Use of this content is subject to our disclaimer