A TIA should be suspected in a patient who presents with sudden-onset, focal neurologic deficit that resolves spontaneously and cannot be explained by another condition such as hypoglycemia.[43]National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Apr 2022 [internet publication].
https://www.nice.org.uk/guidance/ng128
Until the neurologic symptoms and signs have resolved completely, the event should be treated as a stroke, and investigations and management should proceed for this working diagnosis.[43]National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Apr 2022 [internet publication].
https://www.nice.org.uk/guidance/ng128
See Ischemic stroke and Hemorrhagic stroke.
Every patient presenting with acute focal neurologic deficit should receive a rapid history and physical exam, with emphasis on the neurologic exam. An expedited evaluation should occur to determine the necessary workup.
Laboratory testing including complete blood count (CBC), chemistry profile, and blood glucose can help to identify potential mimics of cerebral ischemia.
US guidelines recommend that patients with TIA should preferably undergo neuroimaging evaluation within 24 hours of symptom onset.[1]Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276-93.
https://www.ahajournals.org/doi/full/10.1161/strokeaha.108.192218
http://www.ncbi.nlm.nih.gov/pubmed/19423857?tool=bestpractice.com
In patients suspected of having a stroke or TIA, computed tomography (CT) or magnetic resonance imaging (MRI) of the brain is recommended to confirm the diagnosis.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Typically patients undergo a noncontrast head CT, to exclude a brain hemorrhage and guide treatment.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[44]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Patients with a suspected TIA benefit from early neurology consultation; preferably in the emergency department or via rapid follow-up within 1 week of the TIA.[13]Amin HP, Madsen TE, Bravata DM, et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: a scientific statement from the American Heart Association. Stroke. 2023 Mar;54(3):e109-21.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000418
http://www.ncbi.nlm.nih.gov/pubmed/36655570?tool=bestpractice.com
Evidence suggests that the site at which initial evaluation is provided (e.g., outpatient assessment, hospital emergency department) is not as important as ensuring that the evaluation is completed rapidly, and that all appropriate secondary prevention measures are implemented.[45]Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007 Oct 20;370(9596):1432-42.
http://www.ncbi.nlm.nih.gov/pubmed/17928046?tool=bestpractice.com
Early neurology consultation has been associated with lower 90-day and 1-year mortality rates.[46]Bravata DM, Myers LJ, Reeves M, et al. Processes of care associated with risk of mortality and recurrent stroke among patients with transient ischemic attack and nonsevere ischemic stroke. JAMA Netw Open. 2019 Jul 3;2(7):e196716.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2737105
http://www.ncbi.nlm.nih.gov/pubmed/31268543?tool=bestpractice.com
Hospital admission is warranted if any of the following high-risk features are present:[13]Amin HP, Madsen TE, Bravata DM, et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: a scientific statement from the American Heart Association. Stroke. 2023 Mar;54(3):e109-21.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000418
http://www.ncbi.nlm.nih.gov/pubmed/36655570?tool=bestpractice.com
ABCD2 score ≥4
Subacute stroke on CT
Presumed symptomatic (>50%) extracranial or intracranial stenosis
Infarct on MRI
TIA within the past month
Acute cardiac issues including arrhythmias
Barriers to rapid outpatient follow-up or testing.
In addition, the decision to admit may be influenced by the lack of a reliable observer in the home setting to call emergency medical services in case of second cerebral ischemic event.[47]Johnston SC, Albers GW, Gorelick PB, et al. National Stroke Association recommendations for systems of care for transient ischemic attack. Ann Neurol. 2011 May;69(5):872-7.
http://www.ncbi.nlm.nih.gov/pubmed/21391236?tool=bestpractice.com
Diagnostic steps
Patient presenting with acute neurologic deficit
A focused history to establish time of onset and risk factors for cerebrovascular disease, and to evaluate probability of presence of a mimic of TIA, should be obtained.[44]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
A rapid physical exam with focus on the neurologic exam to determine severity of deficits should be performed.[44]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Laboratory tests including CBC, chemistry profile, blood glucose, prothrombin time (PT), and partial thromboplastin time (PTT) should be done.[44]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
ECG should be performed.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
For rapidly resolving or resolved neurologic deficits referring to a single vascular territory, in the absence of alternative diagnosis to explain symptoms, TIA is the likely diagnosis.
Patients with significant ongoing neurologic deficits should be treated for stroke with consideration of thrombolysis. Therapy should not be delayed in the hope of spontaneous recovery.
CT or MRI of the brain is recommended to confirm the diagnosis in patients suspected of having a stroke or TIA.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Typically patients undergo a noncontrast head CT, to exclude a brain hemorrhage and guide treatment with thrombolysis.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[44]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
MRI of brain with diffusion images is preferred to identify ischemia and potentially to identify distribution of injury.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[13]Amin HP, Madsen TE, Bravata DM, et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: a scientific statement from the American Heart Association. Stroke. 2023 Mar;54(3):e109-21.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000418
http://www.ncbi.nlm.nih.gov/pubmed/36655570?tool=bestpractice.com
However, MRI may take more than 30 minutes to complete, and is not universally available. If MRI is not available, noncontrast CT of the head can be performed. Patients with resolved symptoms may not require a CT scan, and may receive an MRI of the brain, if available. CT and MRI data should be reviewed and interpreted by a physician with expertise in stroke imaging.[44]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
For patients with TIA, follow-up testing should include cardiac monitoring for arrhythmia and echocardiogram to evaluate for cardioembolism, which would be suggested by intracardiac thrombus, valvular vegetation, or atrial fibrillation.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Echocardiography has highest yield when TIA etiology is not evident after the initial evaluation and vascular imaging, or when there is suspicion for a cardioembolic risk factor based on history, ECG, or exam.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Transthoracic echocardiography (TTE) is preferred over transesophageal echocardiography (TEE) for the detection of left ventricular (LV) thrombus, but TOE is superior to TTE in detecting left atrial thrombus, aortic atheroma, prosthetic valve abnormalities, native valve abnormalities, atrial septal abnormalities, and cardiac tumors.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
In patients with ischemic stroke or TIA in whom patent foramen ovale (PFO) closure is contemplated, transcranial Doppler with embolus detection might be reasonable to screen for right-to-left shunt.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Transcranial Doppler compares favorably with TTE for detecting right-to-left shunting, which is usually the result of PFO.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Transcranial Doppler can also be used to identify arterial occlusion of the major arterial branches of the circle of Willis. Spatial resolution is limited compared with CT and MR angiography.
In patients with symptomatic anterior circulation cerebral infarction or TIA who are candidates for revascularization, noninvasive cervical carotid imaging with carotid ultrasonography, CT angiography (CTA), or MR angiography (MRA) is recommended to screen for stenosis of, or plaques within, the intracranial, carotid, or aortic vessels.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Since MRI is relatively rapid, noninvasive and does not required intravenous contrast, MRA may be preferable to CTA in patients with renal impairment, x-ray contrast allergy, or repeat presentations.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
However, noncontrast MRA of the neck tends to overestimate the degree of carotid stenosis when compared with contrast-enhanced MRA, particularly in cases of high-grade stenosis.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[48]Debrey SM, Yu H, Lynch JK, et al. Diagnostic accuracy of magnetic resonance angiography for internal carotid artery disease: a systematic review and meta-analysis. Stroke. 2008 Aug;39(8):2237-48.
https://www.ahajournals.org/doi/10.1161/STROKEAHA.107.509877
http://www.ncbi.nlm.nih.gov/pubmed/18556586?tool=bestpractice.com
High-resolution imaging of the intracranial large arteries and imaging of the extracranial vertebrobasilar arterial system can also be used to identify atherosclerotic disease, dissection, moyamoya disease, or other etiologically relevant vasculopathies.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Transcranial Doppler is performed in rare cases as a complementary test to further evaluate for intracranial stenosis in the absence of CT/MRI.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[49]Mattioni A, Cenciarelli S, Eusebi P, et al. Transcranial Doppler sonography for detecting stenosis or occlusion of intracranial arteries in people with acute ischaemic stroke. Cochrane Database Syst Rev. 2020 Feb 19;2(2):CD010722.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010722.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/32072609?tool=bestpractice.com
A fasting lipid profile is recommended for patients to evaluate for treatable atherosclerotic risk factors.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) should be ordered if central nervous system vasculitis is suspected, but this is not routine in many centers.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
A hypercoagulability panel (acquired or inherited) may be considered for unexplained TIA in a young patient with self-history or family history of unprovoked thrombosis, prior miscarriages, or coexistence of systemic signs and symptoms suggestive of hypercoagulability.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Diagnostic algorithm for transient ischemic attackAdapted from an algorithm supplied by the previous contributor, Dr Ethan Cumbler [Citation ends].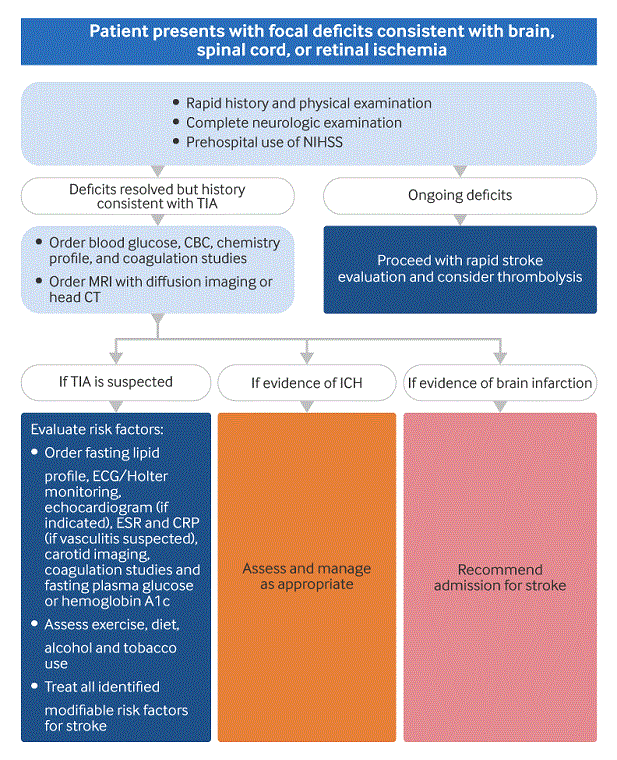
History and physical examination
TIA is fundamentally a clinical diagnosis. Therefore, an accurate history, informed by the patient/caregiver's report of focal neurologic deficit, is key. TIA symptoms are typically brief and the majority will resolve within the first hour. Persistent symptoms at presentation should be treated as stroke, and therapy should not be delayed in the hope of spontaneous recovery.[43]National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Apr 2022 [internet publication].
https://www.nice.org.uk/guidance/ng128
TIAs are more common in middle age and in older people.[1]Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276-93.
https://www.ahajournals.org/doi/full/10.1161/strokeaha.108.192218
http://www.ncbi.nlm.nih.gov/pubmed/19423857?tool=bestpractice.com
Symptoms in a young patient increase the possibility of alternative diagnosis or a less common etiology for ischemia, such as congenital heart disease, extracranial arterial dissections, drug use, hypercoagulability, or paradoxical emboli.
TIAs generally present with loss of power or sensation ("negative symptoms").[50]Nadarajan V, Perry RJ, Johnson J, et al. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. 2014 Feb;14(1):23-31.
https://pn.bmj.com/content/14/1/23.long
http://www.ncbi.nlm.nih.gov/pubmed/24453269?tool=bestpractice.com
Unilateral weakness or sensory deficits as a result of ischemia in the carotid or middle cerebral artery territories are common.[7]Goldstein LB, Bian J, Samsa GP, et al. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med. 2000 Oct 23;160(19):2941-6.
http://www.ncbi.nlm.nih.gov/pubmed/11041901?tool=bestpractice.com
Aphasia can be seen if the area of ischemia includes language centers. Occlusion of the posterior cerebral artery may give homonymous hemianopsia, whereas thrombus in the retinal artery leads to classic amaurosis fugax or monocular visual loss.[51]Tao WD, Liu M, Fisher M, et al. Posterior versus anterior circulation infarction: how different are the neurological deficits? Stroke. 2012 Aug;43(8):2060-5.
https://www.ahajournals.org/doi/10.1161/STROKEAHA.112.652420
http://www.ncbi.nlm.nih.gov/pubmed/22678088?tool=bestpractice.com
Posterior circulation ischemia may lead to symptoms of vertigo, incoordination, cranial nerve deficits, ataxia, or syncope.[7]Goldstein LB, Bian J, Samsa GP, et al. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med. 2000 Oct 23;160(19):2941-6.
http://www.ncbi.nlm.nih.gov/pubmed/11041901?tool=bestpractice.com
Lacunar symptoms tend to give isolated sensory or isolated motor deficits but also a number of less common symptom complexes.[9]Flemming KD, Brown RD, Petty GW, et al. Evaluation and management of transient ischemic attack and minor cerebral infarction. Mayo Clin Proc. 2004 Aug;79(8):1071-86.
http://www.ncbi.nlm.nih.gov/pubmed/15301338?tool=bestpractice.com
Headache can occur after TIA but is not common and suggests alternative etiology for neurologic deficit such as complex migraine, giant cell arteritis (temporal arteritis), or intracranial bleed.[50]Nadarajan V, Perry RJ, Johnson J, et al. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. 2014 Feb;14(1):23-31.
https://pn.bmj.com/content/14/1/23.long
http://www.ncbi.nlm.nih.gov/pubmed/24453269?tool=bestpractice.com
Seizures prior to deficit make partial seizure or post-seizure (Todd's) paralysis a more likely diagnosis than TIA.[50]Nadarajan V, Perry RJ, Johnson J, et al. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. 2014 Feb;14(1):23-31.
https://pn.bmj.com/content/14/1/23.long
http://www.ncbi.nlm.nih.gov/pubmed/24453269?tool=bestpractice.com
Key risk factors include atrial fibrillation, valvular disease, carotid stenosis, intracranial stenosis, congestive heart failure, hypertension, hyperlipidemia, diabetes, cigarette smoking, alcohol abuse, and advanced age.[19]Sacco RL. Risk factors for TIA and TIA as a risk factor for stroke. Neurology. 2004 Apr 27;62(8 Suppl 6):S7-11.
http://www.ncbi.nlm.nih.gov/pubmed/15111649?tool=bestpractice.com
[20]Whisnant JP, Brown RD, Petty GW, et al. Comparisons of population-based models of risk factors for TIA and ischemic stroke. Neurology. 1999 Aug 11;53(3):532-6.
http://www.ncbi.nlm.nih.gov/pubmed/10449116?tool=bestpractice.com
[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
[26]Woo D, Gebel J, Miller R, et al. Incidence rates of first-ever ischemic stroke subtypes among blacks: a population-based study. Stroke. 1999 Dec;30(12):2517-22.
https://www.ahajournals.org/doi/full/10.1161/01.str.30.12.2517
http://www.ncbi.nlm.nih.gov/pubmed/10582971?tool=bestpractice.com
[27]Bots ML, Van der Wilk EC, Koudstaal PJ, et al. Transient neurological attacks in the general population: prevalence, risk factors, and clinical relevance. Stroke. 1997 Apr;28(4):768-73.
https://www.ahajournals.org/doi/full/10.1161/01.str.28.4.768
http://www.ncbi.nlm.nih.gov/pubmed/9099194?tool=bestpractice.com
[28]Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989 Mar 25;298(6676):789-94.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1836102/pdf/bmj00224-0029.pdf
http://www.ncbi.nlm.nih.gov/pubmed/2496858?tool=bestpractice.com
[31]Hillbom M, Saloheimo P, Juvela S. Alcohol consumption, blood pressure, and the risk of stroke. Curr Hypertens Rep. 2011 Jun;13(3):208-13.
http://www.ncbi.nlm.nih.gov/pubmed/21327566?tool=bestpractice.com
[52]Reynolds K, Lewis B, Nolen JD, et al. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003 Feb 5;289(5):579-88. [Erratum in: JAMA. 2003 Jun 4;289(21):2798.]
http://www.ncbi.nlm.nih.gov/pubmed/12578491?tool=bestpractice.com
[53]Larsson SC, Wallin A, Wolk A, et al. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med. 2016 Nov 24;14(1):178.
https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0721-4
http://www.ncbi.nlm.nih.gov/pubmed/27881167?tool=bestpractice.com
Presence of hypertension, diabetes, hyperlipidemia, or chronic renal disease increases the probability of atherosclerotic disease as a risk factor for TIA.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Presence of family history of stroke at a young age suggests a potential heritable risk factor such as familial hyperlipidemia or hypercoagulability.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Personal history of miscarriage or thromboembolic events may also suggest inherited or acquired thrombophilia.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Patients will often have increased blood pressure (BP) on presentation as BP rises acutely after a cerebral ischemic event. The presence of a carotid bruit is not sensitive or specific for significant stenosis.
It is useful to consider four questions when assessing whether a TIA has occurred:
Are the symptoms negative (i.e., a deficit) rather than positive (i.e., paresthesias, visual scotoma)?
A rapid complete neurologic exam is critical for any patient who presents with acute focal deficits. Pre-hospital screening exams such as the Cincinnati, Los Angeles, or ROSIER tools can be used to quickly assess the possibility of stroke/TIA, but lack specificity.[54]Kothari RU, Pancioli A, Liu T, et al. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999 Apr;33(4):373-8.
http://www.ncbi.nlm.nih.gov/pubmed/10092713?tool=bestpractice.com
[55]Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000 Jan;31(1):71-6.
http://www.ncbi.nlm.nih.gov/pubmed/10625718?tool=bestpractice.com
[56]Nor AM, Davis J, Sen B, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol. 2005 Nov;4(11):727-34.
http://www.ncbi.nlm.nih.gov/pubmed/16239179?tool=bestpractice.com
Cincinnati prehospital stroke scale
Opens in new window
[  ]
What is the accuracy of prehospital stroke scales as screening tools for early detection of stroke and transient ischemic attacks (TIAs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2795/fullShow me the answer
]
What is the accuracy of prehospital stroke scales as screening tools for early detection of stroke and transient ischemic attacks (TIAs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2795/fullShow me the answer
The National Institute of Health Stroke Scale (NIHSS) is the preferred method of quantifying the degree of deficits from stroke. The NIHSS will be abnormal during the occurrence of symptoms, but by definition will revert to the pre-TIA score after the symptoms have resolved. ABCD2, ABCD3, or ABCD3-I scores have been used to predict the risk of stroke after TIA; there is some evidence to suggest that the ABCD3-I score (which incorporates imaging of the brain and carotid arteries) is the most effective.[57]Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014 Feb;45(2):418-25.
https://www.ahajournals.org/doi/full/10.1161/strokeaha.113.003077
http://www.ncbi.nlm.nih.gov/pubmed/24335223?tool=bestpractice.com
[58]Kelly PJ, Albers GW, Chatzikonstantinou A, et al. Validation and comparison of imaging-based scores for prediction of early stroke risk after transient ischaemic attack: a pooled analysis of individual-patient data from cohort studies. Lancet Neurol. 2016 Nov;15(12):1238-47.
http://www.ncbi.nlm.nih.gov/pubmed/27751555?tool=bestpractice.com
Evidence shows that risk prediction scores used in isolation are poor at discriminating low and high risk of stroke after TIA.[58]Kelly PJ, Albers GW, Chatzikonstantinou A, et al. Validation and comparison of imaging-based scores for prediction of early stroke risk after transient ischaemic attack: a pooled analysis of individual-patient data from cohort studies. Lancet Neurol. 2016 Nov;15(12):1238-47.
http://www.ncbi.nlm.nih.gov/pubmed/27751555?tool=bestpractice.com
[59]Merwick A, Albers GW, Amarenco P, et al. Addition of brain and carotid imaging to the ABCD² score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol. 2010 Nov;9(11):1060-9.
http://www.ncbi.nlm.nih.gov/pubmed/20934388?tool=bestpractice.com
This has led some national guidelines outside the US, e.g., the National Institute for Health and Care Excellence (NICE) in the UK, to recommend that all people with suspected TIA are considered as potentially high risk for stroke, with specialist assessment and investigation within 24 hours of symptom onset.[43]National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Apr 2022 [internet publication].
https://www.nice.org.uk/guidance/ng128
[
NIH Stroke Score
Opens in new window
]
[
ABCD2 Score to Predict Stroke Risk after TIA
Opens in new window
]
Laboratory studies
These are performed primarily to exclude metabolic or other systemic illness that may mimic cerebral ischemia. Focused laboratory evaluation including a chemistry profile, serum glucose, fasting lipid profile, and CBC is recommended.[13]Amin HP, Madsen TE, Bravata DM, et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: a scientific statement from the American Heart Association. Stroke. 2023 Mar;54(3):e109-21.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000418
http://www.ncbi.nlm.nih.gov/pubmed/36655570?tool=bestpractice.com
[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Every patient with TIA or stroke should be screened for diabetes mellitus by measuring fasting plasma glucose or hemoglobin A1c, or with an oral glucose tolerance test.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Other testing should be dictated by clinical suspicion for alternative etiology. PT, international normalized ratio, and activated PTT can be ordered if the neurologic deficit persists at time of presentation, there is reason to suspect abnormal coagulation (such as liver disease or use of anticoagulant therapy), and thrombolytic therapy for stroke is being considered. Completing these tests in high-risk patients for early second ischemic events could speed future thrombolysis decisions. ESR and CRP may be helpful if other clues suggest temporal arteritis.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
A hypercoagulability blood panel may be indicated in unexplained TIA in a young patient with self-history or family history of unprovoked thrombosis, prior miscarriages, or coexistence of systemic signs and symptoms suggestive of hypercoagulability.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Rare associations, such as underlying malignancy, may be considered as potential underlying etiologies of thrombophilia.
Imaging
Testing should be individualized.
Head CT scan has poor ability to rule out ischemia in TIA or early stroke.[13]Amin HP, Madsen TE, Bravata DM, et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: a scientific statement from the American Heart Association. Stroke. 2023 Mar;54(3):e109-21.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000418
http://www.ncbi.nlm.nih.gov/pubmed/36655570?tool=bestpractice.com
[60]Moreau F, Asdaghi N, Modi J, et al. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: the more you see the more you know. Cerebrovasc Dis Extra. 2013 Oct 8;3(1):130-6.
https://www.karger.com/Article/FullText/355024
http://www.ncbi.nlm.nih.gov/pubmed/24403904?tool=bestpractice.com
However, CT has nearly 100% sensitivity in ruling out hemorrhage.
MRI diffusion imaging will demonstrate restricted diffusion in areas of ischemia, and approximately half of all patients with a clinical diagnosis of TIA will have abnormal findings.[4]Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke. 1999 Jun;30(6):1174-80.
https://www.ahajournals.org/doi/full/10.1161/01.str.30.6.1174
http://www.ncbi.nlm.nih.gov/pubmed/10356095?tool=bestpractice.com
The probability of abnormal imaging findings rises with the duration of clinical symptoms, and one quarter to one half of patients with clinical resolution of symptoms in <24 hours will have permanent infarction on follow-up imaging, thus suggesting these were actually strokes.[61]Gass A, Ay H, Szabo K, et al. Diffusion-weighted MRI for the "small stuff": the details of acute cerebral ischaemia. Lancet Neurol. 2004 Jan;3(1):39-45.
http://www.ncbi.nlm.nih.gov/pubmed/14693110?tool=bestpractice.com
[62]Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack: proposal for a new definition. N Engl J Med. 2002 Nov 21;347(21):1713-6.
http://www.ncbi.nlm.nih.gov/pubmed/12444191?tool=bestpractice.com
Although TIAs may have normal neuroimaging, MRI with diffusion may be helpful in confirming cerebral ischemia or risk stratification for early second events.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
In some cases, areas with infarcts in multiple arterial distributions are seen, which may suggest embolic etiology not suspected by clinical exam. Small posterior fossa ischemic strokes can be missed with diffusion-weighted MRI up to 48 hours after symptom onset.[63]Saber Tehrani AS, Kattah JC, Mantokoudis G, et al. Small strokes causing severe vertigo: frequency of false-negative MRIs and nonlacunar mechanisms. Neurology. 2014 Jul 8;83(2):169-73.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4117176
http://www.ncbi.nlm.nih.gov/pubmed/24920847?tool=bestpractice.com
If diffusion-weighted imaging is negative and there is a strong clinical suspicion of TIA, perfusion-weighted imaging may be performed during the same MRI examination; in 30% of cases, a focal perfusion deficit is identified in the brain area corresponding to the symptoms.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
[3]Amarenco P. Transient ischemic attack. N Engl J Med. 2020 May 14;382(20):1933-41.
http://www.ncbi.nlm.nih.gov/pubmed/32402163?tool=bestpractice.com
[64]Mlynash M, Olivot JM, Tong DC, et al. Yield of combined perfusion and diffusion MR imaging in hemispheric TIA. Neurology. 2009 Mar 31;72(13):1127-33.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2680066
http://www.ncbi.nlm.nih.gov/pubmed/19092109?tool=bestpractice.com
[65]Grams RW, Kidwell CS, Doshi AH, et al. Tissue-negative transient ischemic attack: is there a role for perfusion MRI? AJR Am J Roentgenol. 2016 Jul;207(1):157-62.
https://www.ajronline.org/doi/10.2214/AJR.15.15447
http://www.ncbi.nlm.nih.gov/pubmed/27070836?tool=bestpractice.com
In patients suspected of having had a TIA, if the initial head imaging (CT or MRI) does not demonstrate a symptomatic cerebral infarct, follow-up MRI is reasonable to predict risk of early stroke and to support the diagnosis.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Carotid Doppler ultrasound is a commonly employed screening test for stenosis, but is not useful in posterior circulation TIAs. Intracranial vasculature is not visualized with carotid Doppler.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
In patients with symptomatic anterior circulation cerebral infarction or TIA who are candidates for revascularization, noninvasive cervical carotid imaging with carotid ultrasonography, CT angiography, or MR angiography is recommended to screen for stenosis of, or plaques within, the intracranial, carotid, or aortic vessels.[13]Amin HP, Madsen TE, Bravata DM, et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: a scientific statement from the American Heart Association. Stroke. 2023 Mar;54(3):e109-21.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000418
http://www.ncbi.nlm.nih.gov/pubmed/36655570?tool=bestpractice.com
[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
High-resolution imaging of the intracranial large arteries and imaging of the extracranial vertebrobasilar arterial system can also be effective in identifying atherosclerotic disease, dissection, moyamoya, or other etiologically relevant vasculopathies.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Transcranial Doppler is less commonly used to further evaluate intracranial stenosis suggested on other imaging modalities.[2]American College of Radiology. ACR appropriateness criteria: cerebrovascular diseases-stroke and stroke-related conditions. 2023 [internet publication].
https://acsearch.acr.org/docs/3149012/Narrative
Physiological studies
Telemetry/Holter monitor studies should be performed in all patients with a TIA to evaluate for atrial fibrillation and other arrhythmias.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Extended cardiac monitoring will identify new atrial fibrillation/flutter in significantly more patients than ECG/short-term telemetry monitoring and is an appropriate consideration for patients with unexplained TIA.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
[66]Higgins P, MacFarlane PW, Dawson J, et al. Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke. 2013 Sep;44(9):2525-31.
https://www.ahajournals.org/doi/full/10.1161/strokeaha.113.001927
http://www.ncbi.nlm.nih.gov/pubmed/23899913?tool=bestpractice.com
[67]Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014 Jun 26;370(26):2467-77.
https://www.nejm.org/doi/full/10.1056/NEJMoa1311376
http://www.ncbi.nlm.nih.gov/pubmed/24963566?tool=bestpractice.com
An echocardiogram may be done in the TIA evaluation to look for intracardiac thrombus or valvular disease.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
TTE is preferred over TEE for the detection of LV thrombus, but TEE is superior to TTE in detecting left atrial thrombus, aortic atheroma, prosthetic valve abnormalities, native valve abnormalities, atrial septal abnormalities, and cardiac tumors.[21]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Bubble studies can establish if there are intracardiac shunts for selected patients such as those with TIA under the age of 65 years without risk factors, and patients with cryptogenic TIA or neurologic deficits occurring with Valsalva.[47]Johnston SC, Albers GW, Gorelick PB, et al. National Stroke Association recommendations for systems of care for transient ischemic attack. Ann Neurol. 2011 May;69(5):872-7.
http://www.ncbi.nlm.nih.gov/pubmed/21391236?tool=bestpractice.com
Transcranial Doppler with bubble study may help quantify the magnitude of a right-to-left shunt and can be performed simultaneously with a TTE bubble study.[68]Serena J, Segura T, Perez-Ayuso MJ, et al. The need to quantify right-to-left shunt in acute ischemic stroke: a case-control study. Stroke. 1998 Jul;29(7):1322-8.
https://www.ahajournals.org/doi/10.1161/01.str.29.7.1322
http://www.ncbi.nlm.nih.gov/pubmed/9660381?tool=bestpractice.com

 ]
]