The management of central airway obstruction (CAO) is challenging and requires a multidisciplinary team approach with involvement of a pulmonologist, medical and radiation oncologist, anesthesiologist, ear, nose and throat specialist, thoracic surgeon, and interventional bronchoscopist.[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
CAO management is largely dependent on the initial presentation. More than half of the interventions performed for an airway obstruction are done on an urgent or emergency basis.[28]Ernst A, Simoff M, Ost D, et al. Prospective risk-adjusted morbidity and mortality outcome analysis after therapeutic bronchoscopic procedures: results of a multi-institutional outcomes database. Chest. 2008;134:514-519.
http://www.ncbi.nlm.nih.gov/pubmed/18641088?tool=bestpractice.com
Airway obstruction presenting with imminent suffocation requires immediate action to promptly and effectively re-establish and secure a patent airway and relieve the obstruction.[18]Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258-1271.
http://erj.ersjournals.com/content/27/6/1258.full
http://www.ncbi.nlm.nih.gov/pubmed/16772389?tool=bestpractice.com
Due to the acuity of the presentation in such patients, investigations that would normally be preliminary (e.g., high-resolution computed tomography, pulmonary function tests) and a diagnostic flexible bronchoscopy may not initially be performed.
Most patients whose presentation of CAO is benign or nonacute are treated as day-cases in an outpatient setting. These patients are observed for several hours in the recovery room and, if clinically stable after the procedure, are discharged that day.[9]Mehta AC, Harris RJ, De Boer GE. Endoscopic management of benign airway stenosis. Clin Chest Med. 1995;16:401-413.
http://www.ncbi.nlm.nih.gov/pubmed/8521696?tool=bestpractice.com
Experts generally describe a two-step treatment approach to CAO, with initial stabilization followed by the use of various airway interventions that can be divided into endoscopic, or surgical therapies.
Initial stabilization
The initial step, and a mandatory priority in the management of CAO, is to maintain adequate oxygenation and ventilation.
Patients with a subacute presentation of CAO may be treated with supplemental oxygen via nasal cannulae or a respiratory mask. In such stable patients, additional diagnostic information may be obtained via a diagnostic flexible bronchoscopy.
In patients presenting with severe tracheal or bronchial obstruction who are unstable with impending respiratory failure, initial stabilization is focused on establishing a secure airway. These patients should be evaluated and managed in an intensive care setting. The establishment of a secure airway may require endotracheal intubation or rigid bronchoscopy. In patients with severe proximal upper airway obstruction, urgent cricothyroidotomy or tracheotomy are the procedures of choice.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006.
Endotracheal intubation should be performed with anesthesia of the mucous membranes in an awake or mildly sedated patient who is actively breathing. Avoiding paralytics is advisable, as intubation may be difficult or impossible.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006. Although patients with CAO are very anxious, sedatives should be used with caution as hypoventilation may further compromise the airway.[60]Brodsky JB. Bronchoscopic procedures for central airway obstruction. J Cardiothorac Vasc Anesth. 2003;17:638-646.
http://www.ncbi.nlm.nih.gov/pubmed/14579222?tool=bestpractice.com
[82]Finlayson GN, Brodsky JB. Anesthetic considerations for airway stenting in adult patients. Anesthesiol Clin. 2008;26:281-291.
http://www.ncbi.nlm.nih.gov/pubmed/18456213?tool=bestpractice.com
The endotracheal tube (ETT) must be carefully advanced along the trachea, as trauma to the friable tissues may exacerbate an intraluminal obstruction and cause bleeding.[60]Brodsky JB. Bronchoscopic procedures for central airway obstruction. J Cardiothorac Vasc Anesth. 2003;17:638-646.
http://www.ncbi.nlm.nih.gov/pubmed/14579222?tool=bestpractice.com
Fiberoptic-assisted intubation with ETT placement under direct visualization should be considered for proximal tracheal obstructions.[60]Brodsky JB. Bronchoscopic procedures for central airway obstruction. J Cardiothorac Vasc Anesth. 2003;17:638-646.
http://www.ncbi.nlm.nih.gov/pubmed/14579222?tool=bestpractice.com
A laryngeal mask airway or suspension laryngoscopy are alternative options to avoid these complications.[82]Finlayson GN, Brodsky JB. Anesthetic considerations for airway stenting in adult patients. Anesthesiol Clin. 2008;26:281-291.
http://www.ncbi.nlm.nih.gov/pubmed/18456213?tool=bestpractice.com
If there is any doubt regarding the stability of the airway in severe obstruction, rigid bronchoscopy is the procedure of choice as it provides a secure airway, enabling oxygenation and ventilation.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[70]Jeon K, Kim H, Yu CM, et al. Rigid bronchoscopic intervention in patients with respiratory failure caused by malignant central airway obstruction. J Thorac Oncol. 2006 May;1(4):319-23.
http://www.ncbi.nlm.nih.gov/pubmed/17409877?tool=bestpractice.com
It also serves as a therapeutic tool for rapid stenosis dilation.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006.
Heliox, a mixture of 60% to 80% helium and 20% to 40% oxygen, may be used in acute patients as a bridging therapy, in order to avoid intubation, or to perform a more secure or stable intubation. It reduces the work of breathing by reducing the turbulent flow of gases in the large airways, and allows faster establishment of a laminar flow after changes in airway diameter.[15]Aboussouan LS, Stoller JK. Diagnosis and management of upper airway obstruction. Clin Chest Med. 1994;15:35-53.
http://www.ncbi.nlm.nih.gov/pubmed/8200192?tool=bestpractice.com
[82]Finlayson GN, Brodsky JB. Anesthetic considerations for airway stenting in adult patients. Anesthesiol Clin. 2008;26:281-291.
http://www.ncbi.nlm.nih.gov/pubmed/18456213?tool=bestpractice.com
This effect lowers the driving pressure required to obtain a given flow, or improves flow at the same driving pressure. The resultant reduced work of breathing allows for a more stable intubation with an ETT or rigid bronchoscopy. The major limitation of the use of Heliox is the inability to deliver gas with a fraction of inspired oxygen (FiO2) of >40%. Despite physiologic evidence and clinical reports, prospective randomized trials demonstrating improved outcomes with the use of Heliox are lacking.
If a dedicated airway team is not available, patient transfer to a specialized center should be considered after initial stabilization. If the patient presents with impending respiratory failure due to extrinsic airway compression, immediate intubation using a flexible bronchoscope passed distal to the stenosis and cleaning of the distal airways to remove pus and mucus prior to referral can be life-saving. Inflation of the tracheal cuff aids with the compression of the tumorous section, and the patient can be safely transported to a referral center for further treatment.[18]Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258-1271.
http://erj.ersjournals.com/content/27/6/1258.full
http://www.ncbi.nlm.nih.gov/pubmed/16772389?tool=bestpractice.com
In some cases, extracorporeal membrane oxygenation (ECMO) support may be considered if the degree of stenosis and risk of respiratory decompensation is deemed prohibitive for a conventional bronchoscopic approach.[83]Lin J, Frye L. The intersection of bronchoscopy and extracorporeal membrane oxygenation. J Thorac Dis. 2021 Aug;13(8):5176-82.
https://jtd.amegroups.org/article/view/39924/html
http://www.ncbi.nlm.nih.gov/pubmed/34527357?tool=bestpractice.com
[84]Wu H, Zhuo K, Cheng D. Extracorporeal membrane oxygenation in critical airway interventional therapy: A review. Front Oncol. 2023;13:1098594.
https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2023.1098594/full
http://www.ncbi.nlm.nih.gov/pubmed/37051538?tool=bestpractice.com
The potential therapeutic adverse outcome should outweigh the risk of ECMO itself.[23]Shaller BD, Filsoof D, Pineda JM, et al. Malignant central airway obstruction: what's new? Semin Respir Crit Care Med. 2022 Aug;43(4):512-29.
http://www.ncbi.nlm.nih.gov/pubmed/35654419?tool=bestpractice.com
[85]Ratwani AP, Davis A, Maldonado F. Current practices in the management of central airway obstruction. Curr Opin Pulm Med. 2022 Jan 1;28(1):45-51.
http://www.ncbi.nlm.nih.gov/pubmed/34720097?tool=bestpractice.com
Once initial stabilization is achieved in patients not managed with rigid bronchoscopy, or in patients who do not require an urgent intervention, a detailed and careful flexible bronchoscopy, as well as other additional studies required for diagnosis and treatment planning, may be performed.
Malignant airway obstruction
Resectable tumors
Radical surgical resection with systemic nodal dissection is the standard therapeutic approach in resectable tumors.[18]Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258-1271.
http://erj.ersjournals.com/content/27/6/1258.full
http://www.ncbi.nlm.nih.gov/pubmed/16772389?tool=bestpractice.com
Nonresectable tumors
Malignant CAO normally presents as advanced disease with no chance of curative surgical resection.
In patients with inoperable tumors of the central airway, restoration of airway patency provides palliation and may prolong life, especially in cases of CAO presenting with impending respiratory failure.[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
Interventional bronchoscopy may be indicated prior to chemotherapy or radiation therapy (or when such treatment fails) in the management of nonresectable tumors, and has been shown to improve dyspnea and extubation rates, thus increasing quality of life.[29]Cosano Povedano A, Muñoz Cabrera L, Cosano Povedano FJ, et al. Endoscopic treatment of central airway stenosis: five years' experience. Arch Bronconeumol. 2005;41:322-327.
http://www.ncbi.nlm.nih.gov/pubmed/15989889?tool=bestpractice.com
[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
Bronchoscopic therapy is an alternative to surgery in cases where an otherwise resectable tumor is deemed inoperable due to the high functional or anesthetic risk to the patient.[29]Cosano Povedano A, Muñoz Cabrera L, Cosano Povedano FJ, et al. Endoscopic treatment of central airway stenosis: five years' experience. Arch Bronconeumol. 2005;41:322-327.
http://www.ncbi.nlm.nih.gov/pubmed/15989889?tool=bestpractice.com
Although there are very few cases where nonresectable lung cancers have become operable following interventional bronchoscopic treatments, therapeutic bronchoscopy can be used as a complementary tool in the combined bronchoscopic and surgical management of malignant CAO prior to curative lung surgery.[29]Cosano Povedano A, Muñoz Cabrera L, Cosano Povedano FJ, et al. Endoscopic treatment of central airway stenosis: five years' experience. Arch Bronconeumol. 2005;41:322-327.
http://www.ncbi.nlm.nih.gov/pubmed/15989889?tool=bestpractice.com
[30]Chhajed PN, Eberhardt R, Dienemann H, et al. Therapeutic bronchoscopy interventions before surgical resection of lung cancer. Ann Thorac Surg. 2006;81:1839-1843.
http://www.ncbi.nlm.nih.gov/pubmed/16631682?tool=bestpractice.com
[86]Cavaliere S, Foccoli P, Toninelli C, et al. Laser in lung cancer. An 11-year experience with 2253 applications in 1585 patients. J Bronchology. 1996;3:112-115.
Patients with inoperable lung cancer and symptomatic airway obstruction should be offered therapeutic bronchoscopy with mechanical or thermal ablation, brachytherapy, or stent placement, with the aim of improving dyspnea, cough, hemoptysis, and quality of life.[87]Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e455S-e497S.
https://journal.chestnet.org/article/S0012-3692(13)60305-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/23649452?tool=bestpractice.com
A large retrospective study of more than 800 patients showed that interventional bronchoscopic procedures for severe neoplastic airway obstruction have an 85% success rate.[88]Hespanhol V, Magalhães A, Marques A, et al. Neoplastic severe central airways obstruction, interventional bronchoscopy: a decision-making analysis. J Thorac Cardiovasc Surg. 2013;145:926-932.
http://www.ncbi.nlm.nih.gov/pubmed/23020944?tool=bestpractice.com
Nonmalignant airway obstruction
The management of nonmalignant airway obstruction requires close collaboration with a thoracic surgeon experienced in the reconstruction of complex airway abnormalities.
The treatment of central expiratory airway collapse depends on the severity of the functional impairment, its etiology, the degree of airway narrowing, and the extent of the collapse. Tracheobronchomalacia or symptomatic severe dynamic posterior airway collapse may be managed with conservative therapy such as bronchodilators at standard doses or continuous positive pressure ventilation.[13]Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg. 2007;84:1870-1877.
http://www.ncbi.nlm.nih.gov/pubmed/18036901?tool=bestpractice.com
In low-risk patients with focal tracheal stenosis, surgical resection with primary reanastomosis is the first-line therapy.[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
In patients with central expiratory airway collapse due to tracheobronchomalacia or excessive dynamic airway collapse, temporary airway stenting may be indicated if the patient is a candidate for tracheoplasty as there is the possibility that the symptoms will improve after stenting. A foreign body obstructing the central airway may be removed using a variety of instruments including forceps, grasping hooks or baskets, a Fogarty balloon, or a cryotherapy probe.[72]Ko-Pen W, Mehta AC, Turner JF. Flexible bronchoscopy. 2nd ed. Malden, MA: Blackwell; 2004.
Flexible and rigid bronchoscopy
Bronchoscopic therapy, which may be performed via flexible or rigid bronchoscopy, results in an improvement in symptoms, quality of life, and survival. However, for any therapeutically intended relief of obstruction in symptomatic patients, rigid bronchoscopy is the preferred option.[59]Rosell A, Stratakos G. Therapeutic bronchoscopy for central airway diseases. Eur Respir Rev. 2020 Nov 18;29(158):190178.
https://pmc.ncbi.nlm.nih.gov/articles/PMC9488119
http://www.ncbi.nlm.nih.gov/pubmed/33208484?tool=bestpractice.com
Selection of the appropriate approach among the possible endoscopic interventions (thermal, nonthermal, and radiation) depends on a number of factors, including the acuity of the presentation, the underlying cause and type of lesion, the stability of the patient, the patient's general, cardiac, and pulmonary status, quality of life, overall prognosis, physician expertise, and the technology available.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
A multimodal treatment with the combination of various endoscopic techniques is usually used, as some techniques such as laser therapy or electrosurgery with airway stenting are complementary to one another.[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
In general, endoscopic management involves less risk, discomfort, and morbidity than surgical treatment.[Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosisFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].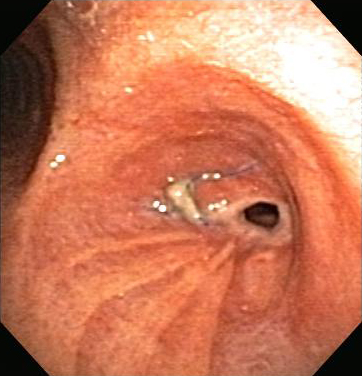 [Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: right mainstem anastomosis post-multimodal endoscopic therapyFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: right mainstem anastomosis post-multimodal endoscopic therapyFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].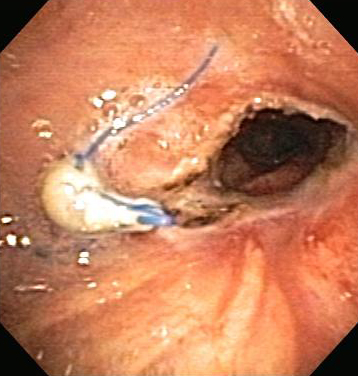
Rigid bronchoscopy
Safe and effective way of securing the airway that provides the ability to ventilate and oxygenate the patient while undertaking diagnostic and therapeutic airway interventions.[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006.[70]Jeon K, Kim H, Yu CM, et al. Rigid bronchoscopic intervention in patients with respiratory failure caused by malignant central airway obstruction. J Thorac Oncol. 2006 May;1(4):319-23.
http://www.ncbi.nlm.nih.gov/pubmed/17409877?tool=bestpractice.com
[71]Ayers ML, Beamis JF Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355-364.
http://www.ncbi.nlm.nih.gov/pubmed/11444118?tool=bestpractice.com
[72]Ko-Pen W, Mehta AC, Turner JF. Flexible bronchoscopy. 2nd ed. Malden, MA: Blackwell; 2004.[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
The bronchoscopic modality of choice in patients with impending respiratory failure and the preferred approach for any therapeutically intended relief of obstruction in symptomatic patients.[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
[59]Rosell A, Stratakos G. Therapeutic bronchoscopy for central airway diseases. Eur Respir Rev. 2020 Nov 18;29(158):190178.
https://pmc.ncbi.nlm.nih.gov/articles/PMC9488119
http://www.ncbi.nlm.nih.gov/pubmed/33208484?tool=bestpractice.com
Allows the use of large-suction catheters to aspirate blood or debris, while the barrel may be used to dilate stenoses and as a coring-out instrument for tumor obstructions.
Requires general anesthesia and an operating theater.[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
Ventilation can be achieved via spontaneous ventilation, spontaneous assisted ventilation, controlled Venturi jet ventilation, high-frequency ventilation, or closed-circuit positive pressure ventilation through the rigid bronchoscope.[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[60]Brodsky JB. Bronchoscopic procedures for central airway obstruction. J Cardiothorac Vasc Anesth. 2003;17:638-646.
http://www.ncbi.nlm.nih.gov/pubmed/14579222?tool=bestpractice.com
[71]Ayers ML, Beamis JF Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355-364.
http://www.ncbi.nlm.nih.gov/pubmed/11444118?tool=bestpractice.com
[82]Finlayson GN, Brodsky JB. Anesthetic considerations for airway stenting in adult patients. Anesthesiol Clin. 2008;26:281-291.
http://www.ncbi.nlm.nih.gov/pubmed/18456213?tool=bestpractice.com
Jet ventilation is used by an open system where a jet ventilation adapter is connected proximally in the rigid bronchoscope. Usually, 100% oxygen is injected at 50 psi with a rate of 8 to 15 breaths per minute. Since it is an open system, room air is also introduced and a variable FiO2 is transmitted to the distal airways. Potential complications include iatrogenic pneumothorax.[90]Yarmus L, Feller-Kopman D. New bronchoscopic instrumentation: a review and update in rigid bronchoscopy. In: Beamis JF, Mathur P, Mehta AC, eds. Interventional pulmonary medicine. 2nd ed. New York, NY: Informa Healthcare; 2009.
Contraindications are those related to anesthesia and the anatomy of the neck and jaw (e.g., unstable cervical spine, oral or maxillofacial trauma, cervical ankylosis, or severe kyphoscoliosis).[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[71]Ayers ML, Beamis JF Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355-364.
http://www.ncbi.nlm.nih.gov/pubmed/11444118?tool=bestpractice.com
Complications are uncommon. Few data exist regarding the incidence of complications, but severe complications are rare in experienced hands. The most common complication is sore throat after the procedure. Other complications include injury to the teeth or gums, tracheal or bronchial tears, and severe bleeding. Hypoxemia-induced cardiac ischemia and arrhythmias are the most dangerous complications. The overall mortality related to rigid bronchoscopy is as low as 0.4%.[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[71]Ayers ML, Beamis JF Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355-364.
http://www.ncbi.nlm.nih.gov/pubmed/11444118?tool=bestpractice.com
[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
[91]Alraiyes AH, Machuzak MS. Rigid bronchoscopy. Semin Respir Crit Care Med. 2014;35:671-680.
http://www.ncbi.nlm.nih.gov/pubmed/25463158?tool=bestpractice.com
A retrospective study has shown that rigid bronchoscopy and mechanical debulking as a sole therapy is safe and successful in up to 83% of cases of central airway tumors.[92]Vishwanath G, Madan K, Bal A, et al. Rigid bronchoscopy and mechanical debulking in the management of central airway tumors: an Indian experience. J Bronchology Interv Pulmonol. 2013;20:127-133.
http://www.ncbi.nlm.nih.gov/pubmed/23609246?tool=bestpractice.com
Improvements have been made to the design of the rigid bronchoscope, to create a more versatile instrument.[93]Yarmus L, Feller-Kopman D. Bronchoscopes of the twenty-first century. Clin Chest Med. 2001;31;19-27.
http://www.ncbi.nlm.nih.gov/pubmed/20172429?tool=bestpractice.com
Commonly used rigid bronchoscopes are the Bryan-Dumon series II and the Karl Storz rigid bronchoscope. The Hemer rigid bronchoscope has a measuring tube connected to the bronchoscope that allows the measurement of inspiratory and expiratory pressures, as well as oxygen and carbon dioxide concentrations.[94]Dutau H, Vandemoortele T, Breen DP. Rigid bronchoscopy. Clin Chest Med. 2013;34:427-435.
http://www.ncbi.nlm.nih.gov/pubmed/23993814?tool=bestpractice.com
Rigid bronchoscopy requires special training. It is underutilized in the United States, as rigid bronchoscopy training is offered in only 4.4% of all pulmonary medicine programs, and 31.3% of pulmonary programs that have an interventional pulmonology service.[91]Alraiyes AH, Machuzak MS. Rigid bronchoscopy. Semin Respir Crit Care Med. 2014;35:671-680.
http://www.ncbi.nlm.nih.gov/pubmed/25463158?tool=bestpractice.com
Flexible bronchoscopy
Once initial stabilization is achieved, a detailed and careful flexible bronchoscopy, as well as other additional studies required for diagnosis and treatment planning, may be performed. This is not necessary in patients who are initially managed with rigid bronchoscopy or in patients who require urgent intervention.
Can be performed under local anesthesia with intravenous sedation, or under general anesthesia.
In flexible bronchoscopy procedures without an endotracheal tube (ETT), ventilation is spontaneous, while in those undertaken through an ETT or laryngeal mask airway, intermittent positive pressure ventilation is required.[60]Brodsky JB. Bronchoscopic procedures for central airway obstruction. J Cardiothorac Vasc Anesth. 2003;17:638-646.
http://www.ncbi.nlm.nih.gov/pubmed/14579222?tool=bestpractice.com
Thermal endoscopic airway interventions
All thermal endoscopic airway interventions can be used with either a rigid or a flexible bronchoscope.
Laser therapy[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[3]Beamis JF Jr. Interventional pulmonology techniques for treating malignant large airway obstruction: an update. Curr Opin Pulm Med. 2005;11:292-295.
http://www.ncbi.nlm.nih.gov/pubmed/15928494?tool=bestpractice.com
[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
[18]Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258-1271.
http://erj.ersjournals.com/content/27/6/1258.full
http://www.ncbi.nlm.nih.gov/pubmed/16772389?tool=bestpractice.com
[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006.[72]Ko-Pen W, Mehta AC, Turner JF. Flexible bronchoscopy. 2nd ed. Malden, MA: Blackwell; 2004.[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
[95]Bolliger CT. Laser bronchoscopy, electrocautery, APC and microdebrider. In: Beamis JF, Mathur P, Mehta AC, eds. Interventional pulmonary medicine. 2nd ed. New York, NY: Informa Healthcare; 2009.[96]Chua AP, Santacruz JF, Gildea TR. Pulmonary complications of cancer therapy and central airway obstruction. In: Davis M, Feyer P, Ortner P, et al, eds. Supportive oncology. 1st ed. Philadelphia, PA: Elsevier Saunders; 2011:309-327.
Laser photoresection refers to the application of laser energy to produce thermal, photodynamic, and electromagnetic changes in living tissues. Several types of laser exist, including Nd:YAG (neodymium:yttrium-aluminum-garnet), CO₂ (carbon dioxide), and Nd:YAP (neodymium:yttrium-aluminum-perovskite). Nd:YAG laser is likely the most widely used type of laser for endobronchial disease.
Nd:YAG is a noncontact or contact technique in which energy from an Nd:YAG laser is applied to the airway tissue for the relief of malignant and nonmalignant CAO. [Figure caption and citation for the preceding image starts]: Central airway obstruction: malignant obstruction of the right mainstemFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].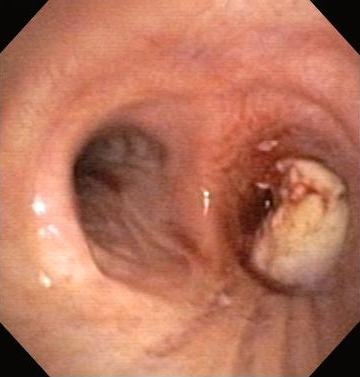 [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: laser photoresectionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: laser photoresectionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].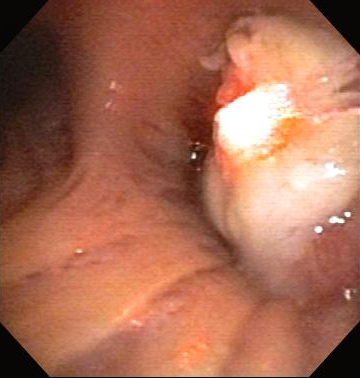
Can be used in emergency situations and is an excellent tool for rapid endobronchial debulking, with a reported rate of lumen restoration of 83% to 93% and symptom relief of 63% to 94%.[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
A “Rule of Four” for laser photoresection can be used to increase the chances of success and minimize risk:[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
Duration of collapse: <4 weeks
Length of lesion: <4 cm
Distance from endotracheal tube to lesion: >4 cm
Distance from fiber tip to lesion (noncontact): 4 mm
Distance from bronchoscope to fiber tip: 4 mm
FiO2: <40%
Power (watts) - noncontact: 40 W
Power (watts) - contact: 4 W
Pulse duration: 0.4 seconds
Number of pulses between cleaning: 40
Operating room time: <4 hours
Laser team: 4
With laser resection, the vaporization of tissue is immediate, and the depth of tissue destruction is usually 3-4 mm. Excellent knowledge of the anatomy is essential to avoid complications such as major vessel perforation.
As the depth of tissue destruction cannot be accurately assessed by the appearance of the tissue surface, extreme caution should be taken to direct the laser beam parallel to the bronchial wall to avoid damage. To achieve an immobile field for accurate alignment of the laser beam, general anesthesia with neuromuscular relaxants is preferred to avoid movements (e.g., coughing).[60]Brodsky JB. Bronchoscopic procedures for central airway obstruction. J Cardiothorac Vasc Anesth. 2003;17:638-646.
http://www.ncbi.nlm.nih.gov/pubmed/14579222?tool=bestpractice.com
[71]Ayers ML, Beamis JF Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355-364.
http://www.ncbi.nlm.nih.gov/pubmed/11444118?tool=bestpractice.com
The only absolute contraindication is isolated extrabronchial disease.
In general, laser resection has a complication rate of <3%.
Complications include perforation (of airways, esophagus, or pulmonary artery), cardiac arrhythmias, pneumothorax (tension and nontension), hemorrhage, hypoxemia, myocardial infarction, stroke, air embolism (secondary to gas exiting the probe tip under pressure and crossing the mucosal membranes into the blood vessels through bronchovascular fistulae formed by coagulation of the tissue), and endobronchial ignition.[61]Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest. 2008;134:1066-1069.
http://www.ncbi.nlm.nih.gov/pubmed/18988782?tool=bestpractice.com
It is thus recommended that the FiO2 should not exceed 40% during the procedure.
Although no randomized trials exist to compare Nd:YAG laser therapy with other forms of CAO management, several retrospective studies have shown successful outcomes.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[97]Dumon JF, Reboud E, Garbe L, et al. Treatment of tracheobronchial lesions by laser photoresection. Chest. 1982;81:278-284.
http://www.ncbi.nlm.nih.gov/pubmed/7056101?tool=bestpractice.com
[98]Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest. 1996;110:1536-1542.
http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21740/1536.pdf
http://www.ncbi.nlm.nih.gov/pubmed/8989073?tool=bestpractice.com
[99]Desai SJ, Mehta AC, VanderBrug Medendorp S, et al. Survival experience following nd:YAG laser photoresection for primary bronchogenic carcinoma. Chest. 1988;94:939-944.
http://www.ncbi.nlm.nih.gov/pubmed/2460297?tool=bestpractice.com
Electrosurgery (electrocautery)[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[3]Beamis JF Jr. Interventional pulmonology techniques for treating malignant large airway obstruction: an update. Curr Opin Pulm Med. 2005;11:292-295.
http://www.ncbi.nlm.nih.gov/pubmed/15928494?tool=bestpractice.com
[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
[18]Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258-1271.
http://erj.ersjournals.com/content/27/6/1258.full
http://www.ncbi.nlm.nih.gov/pubmed/16772389?tool=bestpractice.com
[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
[93]Yarmus L, Feller-Kopman D. Bronchoscopes of the twenty-first century. Clin Chest Med. 2001;31;19-27.
http://www.ncbi.nlm.nih.gov/pubmed/20172429?tool=bestpractice.com
[95]Bolliger CT. Laser bronchoscopy, electrocautery, APC and microdebrider. In: Beamis JF, Mathur P, Mehta AC, eds. Interventional pulmonary medicine. 2nd ed. New York, NY: Informa Healthcare; 2009.[100]Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med. 1999;20:123-138.
http://www.ncbi.nlm.nih.gov/pubmed/10205722?tool=bestpractice.com
[101]Sheski FD, Mathur PN. Endobronchial electrosurgery: argon plasma coagulation and electrocautery. Semin Respir Crit Care Med. 2004;25:367-374.
http://www.ncbi.nlm.nih.gov/pubmed/16088479?tool=bestpractice.com
[102]Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for nd-YAG laser photoresection. Chest. 2000;118:516-521.
http://www.ncbi.nlm.nih.gov/pubmed/10936149?tool=bestpractice.com
Contact or noncontact technique in which a high-frequency alternating electric current is delivered to the airway tissue for the relief of malignant and nonmalignant CAO. [Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosisFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: electrocautery radial incisionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: electrocautery radial incisionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].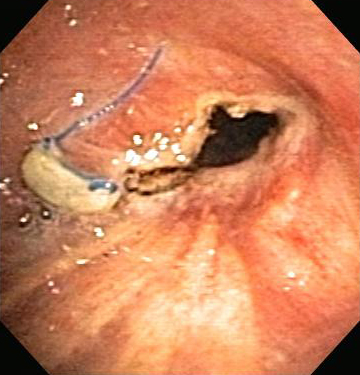
The effect on the airway tissue depends on the power used, the application time, the contact surface area, and the tissue type. The heat generated by the electric current is proportionally related to tissue resistance, and inversely related to tissue vascularity and moisture content.
Can be used in emergency situations and is an excellent tool for rapid endobronchial debulking, with a reported rate of lumen restoration of approximately 90%, and symptom relief of 70% to 97%.
Several tools exist to apply electrocautery in the airway, for example rigid electrocautery probes and forceps. For the flexible bronchoscope, electrocautery snares, knife, blunt probes, and hot forceps are available.
Usually a power setting of 10 W to 40 W is used with the blunt probes and 10 W to 40 W while using the snare or the electrocautery knife.[103]Mahmood K, Wahidi MM. Ablative therapies for central airway obstruction. Semin Respir Crit Care Med. 2014;35:681-692.
http://www.ncbi.nlm.nih.gov/pubmed/25463159?tool=bestpractice.com
Contraindicated in extrinsic airway compression. In patients with pacemakers or automatic implantable cardioverter/defibrillators, due to the potential for dysrhythmias or device malfunction, caution is recommended and the device should be turned off whenever possible and clinically indicated.
Risk of hemorrhage is between 2% and 5%. Other complications include endobronchial ignition, electric shock to the operator if appropriate grounding not in place, and airway perforation. Loss of effectiveness can occur with bleeding due to the diffusion of the current across a larger surface area.
As with laser and argon plasma coagulation, the FiO2 must be below 40% to avoid an airway fire.
Argon plasma coagulation (APC)[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[3]Beamis JF Jr. Interventional pulmonology techniques for treating malignant large airway obstruction: an update. Curr Opin Pulm Med. 2005;11:292-295.
http://www.ncbi.nlm.nih.gov/pubmed/15928494?tool=bestpractice.com
[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
[18]Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258-1271.
http://erj.ersjournals.com/content/27/6/1258.full
http://www.ncbi.nlm.nih.gov/pubmed/16772389?tool=bestpractice.com
[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006.[72]Ko-Pen W, Mehta AC, Turner JF. Flexible bronchoscopy. 2nd ed. Malden, MA: Blackwell; 2004.[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
[93]Yarmus L, Feller-Kopman D. Bronchoscopes of the twenty-first century. Clin Chest Med. 2001;31;19-27.
http://www.ncbi.nlm.nih.gov/pubmed/20172429?tool=bestpractice.com
[95]Bolliger CT. Laser bronchoscopy, electrocautery, APC and microdebrider. In: Beamis JF, Mathur P, Mehta AC, eds. Interventional pulmonary medicine. 2nd ed. New York, NY: Informa Healthcare; 2009.
Noncontact mode of tissue electrocoagulation in which ionized argon gas is used to conduct electric current to the airway tissue in the palliation of malignant CAO as part of multimodal treatment, and for the relief of nonmalignant endobronchial disease such as granulation tissue and airway papillomatosis.[Figure caption and citation for the preceding image starts]: Central airway obstruction: malignant obstruction of the right mainstemFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: argon plasma coagulationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: argon plasma coagulationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].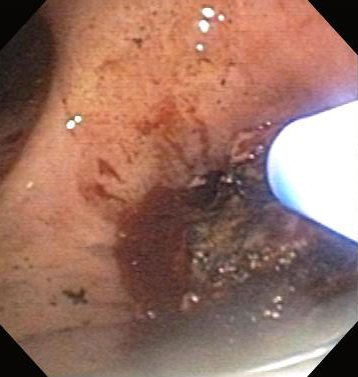
Increasingly being used as an alternative to laser therapy and electrosurgery, as APC is an excellent tool for photocoagulation (hemostasis) with a rate of lumen restoration of 91%.[104]Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119:781-787.
http://www.ncbi.nlm.nih.gov/pubmed/11243957?tool=bestpractice.com
Straight, radial, and lateral fire probes are available for different indications.
Usually a power setting of 30 W is used in the forced mode or 10 W in the pulse mode. The recommended flow of gas is from 0.3 liters per minute to 0.8 liters per minute.[103]Mahmood K, Wahidi MM. Ablative therapies for central airway obstruction. Semin Respir Crit Care Med. 2014;35:681-692.
http://www.ncbi.nlm.nih.gov/pubmed/25463159?tool=bestpractice.com
Although it has the advantage of being able to access lesions lateral to, "around the corner from," and at sharp angles to the probe, APC does not cause tumor vaporization, so other modalities are required for the debulking of large tumor masses. After applying APC to an endobronchial tumor, the operator may remove the resultant eschar and debris with suction, forceps, or cryoadhesion by using the cryoprobe.
Contraindicated in extrinsic airway compression. In patients with pacemakers or automatic implantable cardioverter/defibrillators, due to the potential for dysrhythmias or device malfunction, caution is recommended and the device should be turned off whenever possible and clinically indicated.
Complication rate of <1%. Complications include hemorrhage, airway perforation and stenosis, endobronchial ignition, and air embolism with argon gas. The FiO2 must be below 40% while using APC to avoid airway fires.
Cryotherapy[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
[100]Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med. 1999;20:123-138.
http://www.ncbi.nlm.nih.gov/pubmed/10205722?tool=bestpractice.com
[105]Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest. 1996;110:718-723.
http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21736/718.pdf
http://www.ncbi.nlm.nih.gov/pubmed/8797417?tool=bestpractice.com
Contact technique in which a cryogen (most commonly nitric oxide) is applied to the airway tissue in the treatment of malignant and nonmalignant CAO without impending respiratory failure.
Particularly successful in the removal of foreign objects and blood clots (via cryoadhesion), mucous plugs, granulation tissue, and polypoid lesions, with a rate of lumen restoration of approximately 80%, and symptom relief of 70% to 93%.
Most of the effects of cryosurgery do not occur until some hours after treatment, and historically the use of cryotherapy in the airways has been limited to nonacute or severe airway obstruction. In the literature, most cryotherapy use has been for low-grade stenosis or as adjunctive therapy. The maximal tissue destruction occurs in 1 to 2 weeks, and repeated treatments are recommended to achieve the desired effect.[106]Seaman JC, Musani AI. Endobronchial ablative therapies. Clin Chest Med. 2013;34:417-425.
http://www.ncbi.nlm.nih.gov/pubmed/23993813?tool=bestpractice.com
Case reports and retrospective studies have shown the use of cryotherapy for cryorecanalization to have an immediate treatment effect.[107]Boujaoude Z, Young D, Lotano R, et al. Cryosurgery for the immediate treatment of acute central airway obstruction. J Bronchology Interv Pulmonol. 2013;20:45-47.
http://www.ncbi.nlm.nih.gov/pubmed/23328143?tool=bestpractice.com
A large retrospective study of 225 cases found a 91% success rate with the flexible cryoprobe for cryorecanalization of malignant stenosis, with a safe profile.[108]Schumann C, Hetzel M, Babiak AJ, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg. 2010;139:997-1000.
http://www.jtcvsonline.org/article/S0022-5223%2809%2900877-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/19716140?tool=bestpractice.com
The effect on the airway tissue depends on the number of freezing-thawing cycles, the temperature reached (usually below -40°C), and the water content of the tissue, with maximal effect achieved from rapid freezing and slow thawing. Cryosensitive tissues include the skin, nerves, endothelium, granulation tissue, and mucous membranes. Connective and fibrous tissue, the nerve sheath, cartilage, and fat are cryoresistant.
A safe procedure with few and relatively minor complications, the most common of which are post-procedure fever, and airway sloughing requiring repeat follow-up bronchoscopies. Cryotherapy induces limited damage to the bronchial wall, with no residual stenosis, and has a markedly reduced risk for airway perforation.
Nonthermal endoscopic airway interventions
Photodynamic therapy (PDT)[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
A light of specific wavelength (from a potassium titanyl phosphate [KTP] laser) is applied to the lesion via a flexible bronchoscope 24 to 72 hours after the local or systemic injection of a photosensitizing drug such as dihemato-porphyrin ester (DHE). This leads to a phototoxic reaction and tumor destruction as the photosensitizing drug is preferentially taken up by malignant cells.
Immediately, and 48 hours after the procedure, bronchoscopic toilet (cleaning and debulking of the area to remove tumor debris, retained secretions, and sloughed mucosa) is performed to establish airway patency and assess the necessity of further treatment.
Indicated in the palliative treatment of CAO without acute dyspnea, and particularly useful in distal obstructions due to malignant polypoid endobronchial masses with minimal extrinsic airway compression. Can also be given to patients who have already undergone surgery, radiation, or chemotherapy.
Due to the delayed response of treatment, PDT should not be used in the emergency management of acute, severe CAO.
The most common complication is skin photosensitivity that lasts for 4 to 6 weeks; patients must be advised to avoid sun exposure during this time. Other complications include local airway edema, strictures, hemorrhage, and fistulae formation, although PDT has a lower risk of airway perforation.
The effects are relatively long-lasting, and PDT has been shown to palliate airway obstruction in 80% of patients.
Airway dilation
Dilation of airway stenoses may be achieved with insertion of the barrel of a rigid bronchoscope or with balloon dilation.
Rigid bronchoscopic airway dilation can be used in emergency situations, as rapid recanalization can be achieved.[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
[71]Ayers ML, Beamis JF Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355-364.
http://www.ncbi.nlm.nih.gov/pubmed/11444118?tool=bestpractice.com
The distal end of the rigid bronchoscope acts as a corkscrew dilating a stenosis, or as an apple corer penetrating through large obstructive tumors. The barrel of the bronchoscope can be used to tamponade bleeding lesions. Large forceps may be introduced through the bronchoscope to aid in the mechanical debridement of bulky tumors, to remove foreign bodies, or to evacuate clots. A flexible bronchoscope may be used during rigid bronchoscopy to facilitate tissue debridement in angulated or distal airways. These techniques, although still commonly used, should be reserved for the most severe cases.[28]Ernst A, Simoff M, Ost D, et al. Prospective risk-adjusted morbidity and mortality outcome analysis after therapeutic bronchoscopic procedures: results of a multi-institutional outcomes database. Chest. 2008;134:514-519.
http://www.ncbi.nlm.nih.gov/pubmed/18641088?tool=bestpractice.com
Balloon bronchoplasty (BBP) can be performed during rigid or flexible bronchoscopy with or without fluoroscopy, and involves the use of increasingly larger diameter balloons filled with saline and maintained in position for 15-60 seconds to gently dilate the airway.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[109]Hautmann H, Gamarra F, Pfeifer KJ, et al. Fiberoptic bronchoscopic balloon dilatation in malignant tracheobronchial disease: indications and results. Chest. 2001;120:43-49.
http://www.ncbi.nlm.nih.gov/pubmed/11451814?tool=bestpractice.com
[110]McArdle JR, Gildea TR, Mehta AC. Balloon bronchoplasty: its indications, benefits, and complications. J Bronchology. 2005;12:123-127.
http://journals.lww.com/bronchology/Fulltext/2005/04000/Balloon_Bronchoplasty__Its_Indications,_Benefits,.18.aspx
It induces less mucosal trauma and subsequent granulation tissue formation than does rigid dilation. Balloon tracheoplasty and bronchoplasty can be used in malignant and nonmalignant CAO, stenoses following surgical resections and lung transplantation, and in postintubation tracheal stenosis. [Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: balloon bronchoplastyFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends]. BBP results in an immediate improvement in extrinsic and intrinsic malignant CAO in up to 79% of patients and is useful in airway dilation prior to stenting. As its effects are not long-lasting, BBP dilation is usually followed by other therapies such as laser resection, radiation therapy, or stenting. Complications include stenosis recurrence, pain, mediastinitis, and bleeding, as well as airway tearing or rupture with subsequent pneumothorax or pneumomediastinum.
BBP results in an immediate improvement in extrinsic and intrinsic malignant CAO in up to 79% of patients and is useful in airway dilation prior to stenting. As its effects are not long-lasting, BBP dilation is usually followed by other therapies such as laser resection, radiation therapy, or stenting. Complications include stenosis recurrence, pain, mediastinitis, and bleeding, as well as airway tearing or rupture with subsequent pneumothorax or pneumomediastinum.
Airway stents[3]Beamis JF Jr. Interventional pulmonology techniques for treating malignant large airway obstruction: an update. Curr Opin Pulm Med. 2005;11:292-295.
http://www.ncbi.nlm.nih.gov/pubmed/15928494?tool=bestpractice.com
[111]Santacruz JF, Folch E, Mehta AC. Silicone and metallic stents in interventional pulmonology. Minerva Pneumol. 2009;48:243-259.[112]Casal RF. Update in airway stents. Curr Opin Pulm Med. 2010;16:321-328.
http://www.ncbi.nlm.nih.gov/pubmed/20539232?tool=bestpractice.com
Endobronchial prostheses of various materials can be used to support and maintain patency of the airway. Two types of stent (silicone and metallic or hybrid) are currently widely used in the airway, the most commonly used being the silicone stent, which is inserted with rigid bronchoscopy.
Silicone stents (e.g., Dumon stent, Montgomery T-tube, Hood stent, Reynders-Noppen Tygon stent) are complicated by a high migration rate and obstruction by granulation tissue formation at the stent-ends or by mucous secretions due to impaired mucociliary clearance.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006.[72]Ko-Pen W, Mehta AC, Turner JF. Flexible bronchoscopy. 2nd ed. Malden, MA: Blackwell; 2004.[113]Wood DE, Liu YH, Vallieres E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg. 2003;76:167-172.
http://www.ncbi.nlm.nih.gov/pubmed/12842534?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Chest x-ray showing right mainstem endobronchial stent occlusion with mucusFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
Silicone stents can be used in benign and malignant diseases and are the preferred stent type for benign airway diseases.[1]Murgu SD, Egressy K, Laxmanan B, et al. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest. 2016 Aug;150(2):426-41.
https://journal.chestnet.org/article/S0012-3692(16)00615-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26874192?tool=bestpractice.com
The Dumon-Y silicone stent is particularly useful in malignant diseases involving the carina and the mainstem bronchi. One retrospective analysis has shown the safety and efficacy of a new type of self-expanding metallic Y stent.[114]Gompelmann D, Eberhardt R, Schuhmann M, et al. Self-expanding Y stents in the treatment of central airway stenosis: a retrospective analysis. Ther Adv Respir Dis. 2013;7:255-263.
http://www.ncbi.nlm.nih.gov/pubmed/23823488?tool=bestpractice.com
Hybrid stents, in theory, combine the qualities of silicone and metallic stents (e.g., the covered Wallstent, Ultraflex stent, Polyflex stent, Alveolus stent). They are available in uncovered (metallic stents) and covered (hybrid metallic stents) versions. The covered hybrid stents have the advantage of providing a mechanical barrier to tumor ingrowth.
Indicated in the management of extrinsic malignant compression and the maintenance of airway patency after intrinsic or mixed malignant endoscopic tumor removal.[Figure caption and citation for the preceding image starts]: Central airway obstruction: malignant obstruction of the right mainstemFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: stent placementFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: stent placementFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].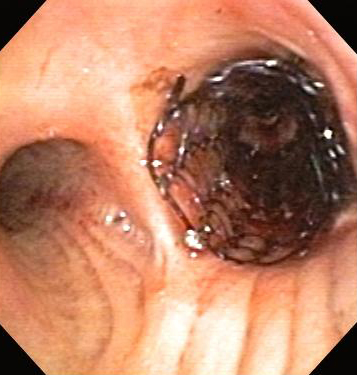 Although used in post-lung transplantation recalcitrant stenosis, stenting is only used in other forms of nonmalignant CAO if other treatments fail, as the complication rate of stenting in such conditions is 75%.[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
[115]Lunn W, Feller-Kopman D, Wahidi M, et al. Endoscopic removal of metallic airway stents. Chest. 2005;127:2106-2112.
http://www.ncbi.nlm.nih.gov/pubmed/15947327?tool=bestpractice.com
[116]Gildea TR, Murthy SC, Sahoo D, et al. Performance of a self-expanding silicone stent in palliation of benign airway conditions. Chest. 2006;130:1419-1423.
http://www.ncbi.nlm.nih.gov/pubmed/17099019?tool=bestpractice.com
Although used in post-lung transplantation recalcitrant stenosis, stenting is only used in other forms of nonmalignant CAO if other treatments fail, as the complication rate of stenting in such conditions is 75%.[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
[115]Lunn W, Feller-Kopman D, Wahidi M, et al. Endoscopic removal of metallic airway stents. Chest. 2005;127:2106-2112.
http://www.ncbi.nlm.nih.gov/pubmed/15947327?tool=bestpractice.com
[116]Gildea TR, Murthy SC, Sahoo D, et al. Performance of a self-expanding silicone stent in palliation of benign airway conditions. Chest. 2006;130:1419-1423.
http://www.ncbi.nlm.nih.gov/pubmed/17099019?tool=bestpractice.com
Although self-expanding metallic stents are relatively easy to deploy without the need of rigid bronchoscopy, their complications may be severe and their use in benign diseases must be with caution. In 2005, the Food and Drug Administration (FDA) also issued a warning that the use of metallic stents should be avoided in benign diseases.
Provide immediate and durable palliation, with symptomatic relief achieved in up to 84% of patients.[117]Saji H, Furukawa K, Tsutsui H, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg. 2010;11:425-428.
http://icvts.oxfordjournals.org/content/11/4/425.full
http://www.ncbi.nlm.nih.gov/pubmed/20656802?tool=bestpractice.com
Tracheobronchial stents have been shown to improve quality of life and survival in patients with advanced malignant obstruction.[113]Wood DE, Liu YH, Vallieres E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg. 2003;76:167-172.
http://www.ncbi.nlm.nih.gov/pubmed/12842534?tool=bestpractice.com
[118]Kim JH, Shin JH, Song HY, et al. Use of a retrievable metallic stent internally coated with silicone to treat airway obstruction. J Vasc Interv Radiol. 2008;19:1208-1214.
http://www.ncbi.nlm.nih.gov/pubmed/18656015?tool=bestpractice.com
[119]Chhajed PN, Baty F, Pless M, et al. Outcome of treated advanced non-small cell lung cancer with and without central airway obstruction. Chest. 2006;130:1803-1807.
http://www.ncbi.nlm.nih.gov/pubmed/17167000?tool=bestpractice.com
[120]Furukawa K, Ishida J, Yamaguchi G, et al. The role of airway stent placement in the management of tracheobronchial stenosis caused by inoperable advanced lung cancer. Surg Today. 2010;40:315-320.
http://www.ncbi.nlm.nih.gov/pubmed/20339985?tool=bestpractice.com
Associated with a high complication rate, particularly with long-term use of metallic or hybrid stents.[19]Makris D, Marquette CH. Tracheobronchial stenting and central airway replacement. Curr Opin Pulm Med. 2007;13:278-283.
http://www.ncbi.nlm.nih.gov/pubmed/17534173?tool=bestpractice.com
In addition to the aforementioned complications, stents may also be associated with halitosis, stent fracture, metal fatigue, airway and vascular perforations, mucosal tears, and obstruction of lobar orifices.[18]Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258-1271.
http://erj.ersjournals.com/content/27/6/1258.full
http://www.ncbi.nlm.nih.gov/pubmed/16772389?tool=bestpractice.com
[67]Simoff MJ, Sterman DH, Ernst A (eds). Thoracic endoscopy. Advances in interventional pulmonology. Malden, MA: Blackwell; 2006.[72]Ko-Pen W, Mehta AC, Turner JF. Flexible bronchoscopy. 2nd ed. Malden, MA: Blackwell; 2004.[121]Mehta AC, Dasgupta A. Airway stents. Clin Chest Med. 1999;20:139-151.
http://www.ncbi.nlm.nih.gov/pubmed/10205723?tool=bestpractice.com
A “stent alert” card should be given to every patient with an airway stent. It should specify the type and size of the stent, the location, and the appropriate size of the endotracheal tube to be used if an emergency intubation is required in the case of tracheal stents.[122]Lee P, Kupeli E, Mehta AC. Airway stents. Clin Chest Med. 2010;31;141-150.
http://www.ncbi.nlm.nih.gov/pubmed/20172440?tool=bestpractice.com
Microdebrider[95]Bolliger CT. Laser bronchoscopy, electrocautery, APC and microdebrider. In: Beamis JF, Mathur P, Mehta AC, eds. Interventional pulmonary medicine. 2nd ed. New York, NY: Informa Healthcare; 2009.[123]Kennedy MP, Morice RC, Jimenez CA, et al. Treatment of bronchial airway obstruction using a rotating tip microdebrider: a case report. J Cardiothorac Surg. 2007;2:16.
http://www.cardiothoracicsurgery.org/content/2/1/16
http://www.ncbi.nlm.nih.gov/pubmed/17386099?tool=bestpractice.com
[124]Lunn W, Bagherzadegan N, Munjampalli SKJ, et al. Initial experience with a rotating airway microdebrider. J Bronchology. 2008;15:91-94.
http://journals.lww.com/bronchology/Fulltext/2008/04000/Initial_Experience_With_a_Rotating_Airway.7.aspx
A powered rotating blade results in accurate debridement of the obstruction. Simultaneous suction allows rapid removal of blood and debris with minimal trauma to the airway, although the tissue removed may not be fit for pathologic inspection.
The microdebrider blade may be smooth or serrated and comes in 2 lengths: 37 cm to access lesions of the trachea and the most proximal main bronchi, and 45 cm for more distal lesions. The usual speed of the blade is between 1000 and 2000 rpm.
Can be used for the therapy of subglottic stenosis as well as granulation tissue and malignant diseases in the trachea, mainstem bronchi, and distal bronchi.[Figure caption and citation for the preceding image starts]: Central airway obstruction: malignant obstruction of the right mainstemFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: post-mechanical debulkingFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: post-mechanical debulkingFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].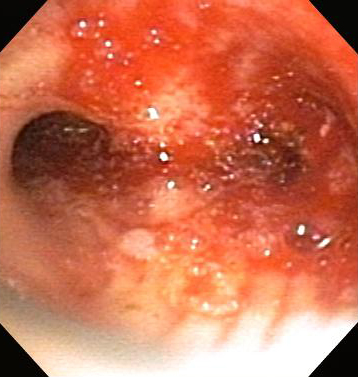
It can be a good option in patients with poor pulmonary reserve since there is no need to decrease the FiO2 during tumor debulking.
The addition of electrocautery may be necessary to achieve hemostasis in up to 35% of patients.
One retrospective study found that the microdebrider is safe and effective in the management of benign and malignant central airway obstruction.[125]Casal RF, Iribarren J, Eapen G, et al. Safety and effectiveness of microdebrider bronchoscopy for the management of central airway obstruction. Respirology. 2013;18:1011-1015.
http://www.ncbi.nlm.nih.gov/pubmed/23520982?tool=bestpractice.com
Radiation (brachytherapy) endoscopic airway interventions
Endobronchial delivery of radiation is achieved via the placement of a radioactive substance (most commonly iridium-192) directly into or in close proximity to the airway tumor using a flexible bronchoscope. This results in tissue destruction through DNA mutations leading to cell apoptosis.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[16]Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society. Eur Respir J. 2002;19:356-373.
http://erj.ersjournals.com/content/19/2/356.full
http://www.ncbi.nlm.nih.gov/pubmed/11866017?tool=bestpractice.com
[17]Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med. 2008 Aug;29(4):441-52.
http://www.ncbi.nlm.nih.gov/pubmed/18651361?tool=bestpractice.com
[22]Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740-749.
http://www.ncbi.nlm.nih.gov/pubmed/11236779?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
[89]Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717.
http://www.ncbi.nlm.nih.gov/pubmed/12740291?tool=bestpractice.com
[100]Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med. 1999;20:123-138.
http://www.ncbi.nlm.nih.gov/pubmed/10205722?tool=bestpractice.com
Brachytherapy is indicated in the palliation of symptoms (in particular dyspnea, cough, and hemoptysis) related to airway obstruction. High-dose endobronchial brachytherapy is also successful in the treatment of excessive granulation tissue formation after lung transplantation at the anastomosis site or as a complication of airway stenting.[14]Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6:79-93.
http://www.ncbi.nlm.nih.gov/pubmed/19131533?tool=bestpractice.com
[126]Kennedy AS, Sonett JR, Orens JB, et al. High dose rate brachytherapy to prevent recurrent benign hyperplasia in lung transplant bronchi: theoretical and clinical considerations. J Heart Lung Transplant. 2000;19:155-159.
http://www.ncbi.nlm.nih.gov/pubmed/10703691?tool=bestpractice.com
[127]Tendulkar RD, Fleming PA, Reddy CA, et al. High-dose-rate endobronchial brachytherapy for recurrent airway obstruction from hyperplastic granulation tissue. Int J Radiat Oncol Biol Phys. 2008;70:701-706.
http://www.ncbi.nlm.nih.gov/pubmed/17904764?tool=bestpractice.com
As brachytherapy takes up to 3 weeks to be effective, it should not be used in the emergency management of acute, severe CAO. The effects of brachytherapy are long-lasting, with a reported rate of lumen restoration of 78% to 85% and symptom relief of 69% to 93%.[7]Manali ED, Saad CP, Krizmanich G, et al. Endobronchial findings of fibrosing mediastinitis. Respir Care. 2003;48:1038-1042.
http://www.rcjournal.com/contents/11.03/11.03.1038.pdf
http://www.ncbi.nlm.nih.gov/pubmed/14635620?tool=bestpractice.com
An advantage of brachytherapy is that it can be used for tumors in areas (e.g., the upper lobe bronchi and segmental bronchi) not accessible to other treatment modalities. The delivery of brachytherapy is via low-dose-rate (LDR) or high-dose-rate (HDR) endobronchial methods. High-dose endobronchial brachytherapy may deliver higher radiation doses with less time on each fraction, thus allowing it to be used in an outpatient setting.
Brachytherapy can be used in combination with other techniques such as laser therapy or external beam radiation, with which it has synergistic effects.
Complications of this technique include hemorrhage (in particular in the right and left upper lobes and often presenting with massive hemoptysis), fistula formation to the mediastinum, arrhythmias, hypotension, bronchospasm, bronchial stenosis or necrosis, and radiation bronchitis. Fatal hemorrhage has been described in up to 32% of cases; however, it is difficult to distinguish between bleeding caused by radiation and that caused by the tumor itself.
External beam radiation (EBR)
Although an established therapy for lung cancer and considered the treatment of choice for patients with inoperable non-small-cell lung cancer, EBR is only variably effective for malignant CAO, and its effects are delayed and unreliable.
With the recent advances in interventional bronchoscopy, the treatment of patients with malignant CAO is shifting from EBR to bronchoscopy, and in centers with interventional pulmonology capabilities, the latter should now be considered as the first-line treatment.[2]Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97.
http://www.ncbi.nlm.nih.gov/pubmed/15187010?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[63]Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261-274.
http://www.ncbi.nlm.nih.gov/pubmed/17218585?tool=bestpractice.com
Occasionally, EBR can be performed in a stable patient with an improved performance status following bronchoscopic intervention in order to consolidate the effects of this treatment.
The major limiting factor of EBR is the unwanted radiation exposure of normal tissue, including the lung parenchyma, heart, spine, and esophagus. Approximately 50% of patients treated with EBR for local control develop disease progression in the irradiated field.
The success rate of EBR therapy for hemoptysis is 84%, although it is only effective in the treatment of airway obstruction and atelectasis in approximately 20% of patients.
Surgical management
Surgery may be indicated in malignant and nonmalignant CAO, depending on the extent of malignant disease at presentation, the nature of the benign disease, and the existence of comorbid medical conditions.[58]Mahmood K, Frazer-Green L, Gonzalez AV, et al. Management of central airway obstruction: an American College of Chest Physicians clinical practice guideline. Chest. 18 Jul 2024 [Epub ahead of print].
https://journal.chestnet.org/article/S0012-3692(24)04614-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/39029785?tool=bestpractice.com
[70]Jeon K, Kim H, Yu CM, et al. Rigid bronchoscopic intervention in patients with respiratory failure caused by malignant central airway obstruction. J Thorac Oncol. 2006 May;1(4):319-23.
http://www.ncbi.nlm.nih.gov/pubmed/17409877?tool=bestpractice.com
[128]Husain SA, Finch D, Ahmed M, et al. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg. 2007;83:1251-1256.
http://www.ncbi.nlm.nih.gov/pubmed/17383321?tool=bestpractice.com
The surgical management of benign CAO is a highly specialized field and should be performed by a thoracic surgeon with significant experience in complex airway procedures.[129]Grillo HC, Mathisen DJ, Wain JC. Management of tumors of the trachea. Oncology (Williston Park). 1992;6:61-67.
http://www.ncbi.nlm.nih.gov/pubmed/1313276?tool=bestpractice.com
The goal of surgery is either to increase the size of the available airway or to resect the stenotic segment. Thus the most common procedures are end-to-end anastomosis or tracheal sleeve resection.
Malignant airway obstruction
Surgery is the treatment of choice for early-stage non-small-cell lung cancer (NSCLC), with a >70% 5-year survival observed in stage IA disease.[30]Chhajed PN, Eberhardt R, Dienemann H, et al. Therapeutic bronchoscopy interventions before surgical resection of lung cancer. Ann Thorac Surg. 2006;81:1839-1843.
http://www.ncbi.nlm.nih.gov/pubmed/16631682?tool=bestpractice.com
[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[130]Hanley ME, Welsh CH. Current diagnosis & treatment in pulmonary medicine. New York, NY: McGraw-Hill; 2003. As 80% of patients present with stage III or IV, the overall 5-year survival is only 4%.[62]Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer: laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241-256.
http://www.ncbi.nlm.nih.gov/pubmed/11901914?tool=bestpractice.com
[130]Hanley ME, Welsh CH. Current diagnosis & treatment in pulmonary medicine. New York, NY: McGraw-Hill; 2003.[131]Schuurmans MM, Michaud GC, Diacon AH, et al. Use of an ultrathin bronchoscope in the assessment of central airway obstruction. Chest. 2003;124:735-739.
http://www.ncbi.nlm.nih.gov/pubmed/12907567?tool=bestpractice.com
Thus, for most advanced lung cancer patients, management is focused on palliation of symptoms and improvement of quality of life.
Nonmalignant airway obstruction
Although surgical resection and anastomosis is the treatment of choice in benign tracheal strictures, tracheal resection is only possible in approximately 50% of patients who are otherwise suitable for surgery, and no satisfactory prosthesis has yet been developed that permits a more extensive tracheal resection.[128]Husain SA, Finch D, Ahmed M, et al. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg. 2007;83:1251-1256.
http://www.ncbi.nlm.nih.gov/pubmed/17383321?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: right mainstem anastomosis post-multimodal endoscopic therapyFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: right mainstem anastomosis post-multimodal endoscopic therapyFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: laser photoresectionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: laser photoresectionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: electrocautery radial incisionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Post-lung transplant anastomotic bronchial stenosis: electrocautery radial incisionFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: argon plasma coagulationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: argon plasma coagulationFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
 BBP results in an immediate improvement in extrinsic and intrinsic malignant CAO in up to 79% of patients and is useful in airway dilation prior to stenting. As its effects are not long-lasting, BBP dilation is usually followed by other therapies such as laser resection, radiation therapy, or stenting. Complications include stenosis recurrence, pain, mediastinitis, and bleeding, as well as airway tearing or rupture with subsequent pneumothorax or pneumomediastinum.
BBP results in an immediate improvement in extrinsic and intrinsic malignant CAO in up to 79% of patients and is useful in airway dilation prior to stenting. As its effects are not long-lasting, BBP dilation is usually followed by other therapies such as laser resection, radiation therapy, or stenting. Complications include stenosis recurrence, pain, mediastinitis, and bleeding, as well as airway tearing or rupture with subsequent pneumothorax or pneumomediastinum.
 [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: stent placementFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: stent placementFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends]. Although used in post-lung transplantation recalcitrant stenosis, stenting is only used in other forms of nonmalignant CAO if other treatments fail, as the complication rate of stenting in such conditions is 75%.[58][115][116]
Although used in post-lung transplantation recalcitrant stenosis, stenting is only used in other forms of nonmalignant CAO if other treatments fail, as the complication rate of stenting in such conditions is 75%.[58][115][116] [Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: post-mechanical debulkingFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Bronchoscopic therapy for central airway obstruction of the right mainstem: post-mechanical debulkingFrom the collections of Jose Fernando Santacruz MD, FCCP, DAABIP and Erik Folch MD, MSc; used with permission [Citation ends].