Tests
1st tests to order
esophagogastroduodenoscopy (EGD) with biopsy
Test
EGD is the first test in patients with severe dysphagia, odynophagia, or weight loss.[88] This will differentiate esophageal cancer from benign causes of dysphagia.[Figure caption and citation for the preceding image starts]: Endoscopic view of esophageal cancerPersonal collection of Mark J. Krasna [Citation ends].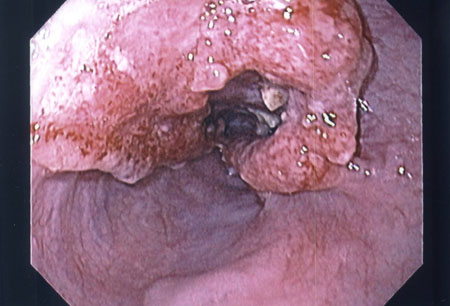
Confocal laser endoscopy with targeted biopsy can improve the diagnostic yield for neoplasia and decrease the number of mucosal biopsies in patients undergoing surveillance.[116][117][118]
Result
mucosal lesion; histology shows squamous cell carcinoma or adenocarcinoma
endoscopic ultrasound (EUS) ± fine needle aspiration (FNA)
Test
Should be performed before treatment for initial clinical staging of the cancer.[15] EUS can identify all the histologic layers of the esophagus and thereby confirm the T stage. The sensitivity and specificity of EUS for staging esophageal cancer has been reported to be: 81.6% and 99.4% in T1 tumors; 81.4% and 96.3% in T2 tumors; 91.4% and 94.4% in T3 tumors; and 92.4% and 97.4% in T4 tumors, respectively.[102]
EUS can identify abnormal or enlarged mediastinal and celiac axis lymph nodes, and enable cytologic exam by FNA. The overall accuracy of EUS/FNA for locoregional staging of esophageal cancer prior to therapy is 87%; the comparable figure for EUS alone is 74%.[100][101][Figure caption and citation for the preceding image starts]: Endoscopic ultrasound-guided fine needle aspiration of lymph nodePersonal collection of Mark J. Krasna [Citation ends].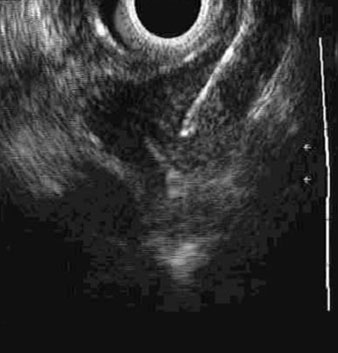
Complications of EUS (with or without FNA) include perforation (0.02% to 0.08%), hemorrhage (0.13% to 0.69%), and infection (0.4% to 1.7%).[107]
Result
indicates extent of local invasion and presence or absence of spread to lymph nodes
CT thorax and abdomen
Test
CT scan of the chest and abdomen is often performed if the suspicion of esophageal cancer is high or biopsy confirms the diagnosis.[89]
CT is most helpful to assess tumor bulk and visceral metastases.
CT also can help identify the thickness of the esophageal lesion and presence of spread to lymph nodes.[Figure caption and citation for the preceding image starts]: CT scan showing T3 tumor at level of inferior pulmonary veinPersonal collection of Mark J. Krasna [Citation ends].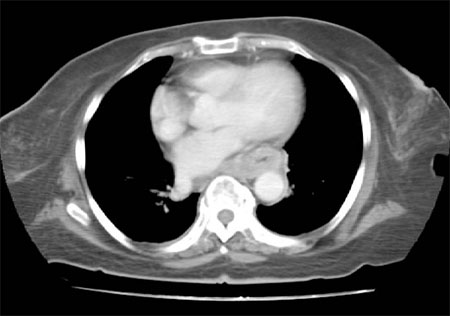
Oral and intravenous contrast should be used to ensure optimal opacification of the lumen and visualization of the heart, mediastinal vessels, and liver.[89]
Result
indicates size of primary tumor, local invasion, and presence or absence of metastases
(18F)-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan
Test
Improves the accuracy of staging and facilitates selection of patients for surgery, by detecting distant metastatic disease not identified by CT alone.[89][Figure caption and citation for the preceding image starts]: PET scan showing esophageal cancer at the gastroesophageal junction. Note metastatic deposit in left femurPersonal collection of Mark J. Krasna [Citation ends].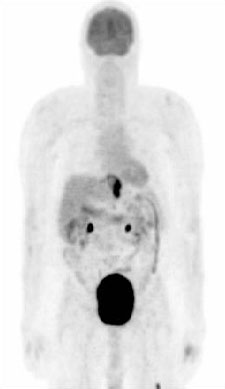
PET can be used to detect responses to chemotherapy and radiation therapy.[94][95][96] In patients with esophageal cancer, relative changes in FDG uptake can predict response to neoadjuvant therapy.[97][98]
An FDG-PET scan is generally done before endoscopic ultrasound (EUS) to avoid unnecessary testing in patients with metastatic disease.[99]
Result
hyperactivity (hot spot) at primary tumor site and locoregional disease; may also show activity in lungs, liver, or bones suggestive of metastases
molecular and pathologic tests
Test
Should be carried out at diagnosis to determine suitability for targeted therapies or immunotherapy. Testing is carried out on tumor tissue obtained at biopsy.
Microsatellite instability (MSI) and/or mismatch repair (MMR) testing
All newly diagnosed patients should be tested for MSI or mismatch repair deficiency (dMMR). Programmed death ligand 1 (PD-L1) testing is recommended in those with advanced or metastatic esophageal cancers.
Testing is performed on formalin-fixed, paraffin-embedded (FFPE) tissue. MSI status is assessed by polymerase chain reaction (PCR) or next-generation sequencing (NGS) to measure gene expression levels of microsatellite markers (BAT25, BAT26, MONO27, NR21, NR24). MMR deficiency is evaluated by immunohistochemistry (IHC) to assess nuclear expression of proteins involved in DNA mismatch repair (MLH1, MSH2, MSH6, PMS2).[15] Results are interpreted as MSI-high (MSI-H) or MMR-deficient (dMMR, deficient mismatch repair) as per the College of American Pathologists (CAP) DNA mismatch repair biomarker reporting guidelines.[111]
Human epidermal receptor 2 (HER2) status
All patients with locally advanced, recurrent, or metastatic inoperable esophageal adenocarcinoma should have HER2 status determined on diagnosis, as HER2-positive patients benefit from the addition of trastuzumab to first-line palliative chemotherapy.[15][114] HER2-targeting is not routine practice in earlier disease settings, and consequently, establishing HER2 status would not impact clinical management outside the context of clinical trials.
HER2 status is assessed using immunohistochemistry (IHC) ± in-situ hybridization (ISH). An alternative is next-generation sequencing (NGS), which offers the opportunity to assess numerous mutations simultaneously.[15]
The reported rates of HER2 positivity in esophageal cancers vary widely (2% to 45%) and are more frequently seen in adenocarcinoma (15% to 30%) than in squamous cell carcinoma (5% to 13%).[15]
Programmed death-ligand 1 (PD-L1) testing
May be considered on locally advanced, recurrent, or metastatic esophageal and esophagogastric junction cancers in patients who are candidates for treatment with PD-1 inhibitors. A qualitative IHC assay is used to detect PD-L1 protein levels in FFPE tumor tissue.
The combined positive score (CPS) is used to evaluate if a specimen is considered to have PD-L1 expression. The CPS is determined by the number of PD-L1 stained cells (i.e., tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells evaluated, multiplied by 100. A CPS ≥1 indicates that a specimen has PD-L1 expression.[15][112] An alternative is the tumor proportion score (TPS): this evaluates the percentage of viable tumor cells showing partial or complete membrane staining at any intensity (PD-L1 positivity is defined as TPS ≥1%).[88] TPS is only used in clinical-decision making for metastatic squamous cell carcinoma (based on CheckMate 648), whereas CPS is used in esophageal and junctional adenocarcinoma and squamous cell carcinoma.[113]
Result
HER2-positive or HER2-negative; MSI-high (MSI-H) or MSI-low (MSI-L) or MSI-stable (MSS); MMR-deficient (dMMR) or no evidence of deficient mismatch repair; PD-L1 expression: positive (CPS ≥1 or TPS ≥1%) or negative (CPS <1 or TPS <1%)
Tests to consider
comprehensive metabolic panel
Test
Should be performed in advanced cases with near or complete esophageal obstruction. These patients may become severely volume-depleted and hypokalemic because of their inability to swallow fluids and their own potassium-rich saliva.
Result
advanced cases: hypokalemia, elevated creatinine and BUN
MRI thorax and abdomen
Test
An alternative to CT for the staging of esophageal cancer, particularly in detecting metastatic disease to visceral organs.
Highly accurate when assessing the liver or adrenals and for determining advanced local spread (T4).
Less reliable in defining early infiltration (T1 to T3).
Appears to be sensitive in predicting mediastinal invasion; the loss of signal in the vessels and the air-filled trachea and bronchi may provide a clear delineation between the tumor and the aorta and the tracheobronchial tree.
Like CT scans, MRI scans are poor at detecting tumors restricted to mucosa or submucosa, and also tend to understage the regional lymph nodes.[92]
Result
indicates size of primary tumor, local invasion, and presence or absence of metastases
bronchoscopy ± fine needle aspiration (FNA)
Test
In patients with disease of the middle and upper thirds of the esophagus, bronchoscopy with FNA can be helpful in determining involvement of the tracheobronchial tree. Bronchoscopy should be performed prior to considering surgery on tumors at these locations.
FNA can be performed into mucosal lesions within the lumen, or transbronchially into lesions adjacent to the airway.[Figure caption and citation for the preceding image starts]: Tracheal invasion (T4) confirmed by bronchoscopyPersonal collection of Mark J. Krasna [Citation ends].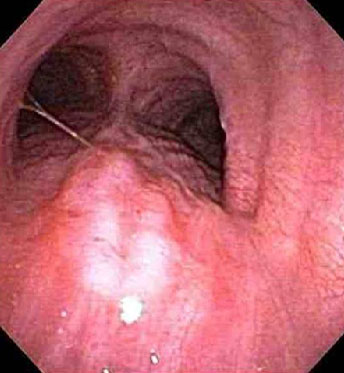
Result
normal or may show involvement of tracheobronchial tree
thoracoscopy and laparoscopy
Test
May be appropriate in selected patients; for example, those with adenocarcinoma of the esophagus or esophagogastric junction with significant extension into the cardia.[108] Studies indicate that thoracoscopy and laparoscopy may improve accuracy compared with noninvasive testing in these situations.[109][110]
Result
may reveal metastatic disease
liquid biopsy
Test
Involves evaluating circulating tumor DNA (ctDNA) by means of a blood test. It is used to detect mutations in DNA shed from esophageal cancer, which can help to identify alterations that are targetable by available treatments. It is being used more frequently in patients with advanced or metastatic disease, especially those who are unable to have a clinical biopsy for disease surveillance and management. A negative result does not exclude the presence of tumor mutations or amplifications and should therefore be interpreted with caution.[15]
Result
ctDNA-positive or negative
pulmonary function tests
Test
Crucial to determine the ability of the patient to withstand combined modality therapy. In patients with poor pulmonary function test results, a less invasive surgical approach, such as transhiatal esophagectomy without thoracotomy, may be associated with lower morbidity and mortality.
Result
normal or reduced
cardiac stress test
Test
Helpful to identify underlying cardiac abnormalities prior to surgery.
Result
normal or may show cardiac abnormality
echocardiogram
Test
Helpful to identify underlying cardiac abnormalities prior to surgery.
Result
normal or may show cardiac abnormality
Use of this content is subject to our disclaimer