Approach
The diagnosis of an IAA is usually suspected from the patient's clinical history, physical examination, and laboratory data. It is confirmed by radiological studies such as computed tomography (CT) scan.[19]
The diagnosis is highly suggested based on timing, when clinical symptoms persist for more than 5 days after abdominal surgery. Many post-operative patients report a recovery period after surgery, followed by a return of general malaise accompanied by fever, abdominal pain, and in some cases, nausea, vomiting, diarrhoea, or severe obstipation.
The location of the abscess is sometimes related to the inciting event. When anastomotic leak or bowel perforation occurs, the abscess usually starts in close proximity to the affected bowel, then propagates depending on the size of the perforation, the severity of the leak, and the time to diagnosis.
History
Patients with IAA have usually had surgery or a recent trauma. They may report a prolonged ileus or abnormal bowel function, either obstipation or diarrhoea. Patients may occasionally relate a period of feeling better after surgery followed by a gradual or sudden decline.
Symptoms of IAA include rapid-onset abdominal pain, loss of appetite, nausea, vomiting, bloating, obstipation, and/or fever.[19] Pain may be masked either by the effects of the surgical incision, or by post-operative pain control. Pain will worsen with time if the patient does not obtain the appropriate medical or surgical care.
IAA can also present without fever or significant abdominal pain, depending on the size, degree of containment, and patient immune system integrity.[3] Patients with a suppressed immune system, caused by disease or medication, frequently present with atypical symptoms that are often mild, despite significant disease burden.
Pelvic abscesses can cause direct irritation to nearby organs, often resulting in dysuria, increased urinary frequency, diarrhoea, and tenesmus. Urinalysis in the setting of pelvic abscesses often demonstrates sterile pyuria secondary to external irritation from the adjacent abscess.
Physical examination
IAA is often associated with fever.[3] Physical examination usually reveals some tenderness overlying the abdominal abscess or a generalised tenderness when multiple abscesses are present. Rebound tenderness may or may not be present. A mass can sometimes be palpated and could be the presenting sign of an appendiceal perforation with abscess formation.[5]
Suspected sepsis
Depending on the individual systemic inflammatory response, patients may present with sepsis or septic shock. Sepsis may also occur early after drainage of an IAA. In those who delay presentation for medical evaluation, intraperitoneal abscesses have been noted to spread out through the skin and subcutaneous tissues. This is often the case where there is an area of soft tissue already traumatised by prior instrumentation, surgical drains, fistula tracts, or tumour involvement.
Early recognition of sepsis is essential because early treatment - when sepsis is suspected but is yet to be confirmed - is associated with significant short- and long-term benefits in outcome.[20] The key to early recognition is the systematic identification of any patient who has signs or symptoms suggestive of infection and is at risk of deterioration due to organ dysfunction. Several risk stratification approaches have been proposed. All rely on a structured clinical assessment and recording of the patient’s vital signs.[20][21][22] It is important to check local guidance for information on which approach your institution recommends.
Laboratory studies
Laboratory studies are not very helpful in the diagnosis of an IAA. Although most commonly IAA are associated with an elevated leukocyte count, a normal white blood cell count should not exclude the diagnosis of an IAA. It is important to consider radiological diagnostic imaging if clinical suspicion of IAA is high, based on the patient's clinical presentation.
Erythrocyte sedimentation rate and C-reactive protein are both non-specific markers of inflammation and could be elevated in patients with IAA.
Once IAA is identified and drained, obtaining a Gram stain and culture (aerobic and anaerobic) is particularly important in higher-risk patients, and patients with hospital-acquired IAA, to identify potential resistant or opportunistic pathogens.[2] Patients with IAA have early empirical antimicrobial therapy initiated after diagnosis, but culture and antimicrobial susceptibility information obtained from cultures of the IAA at the time of drainage permits de-escalation of antimicrobial therapy.
Serum glucose test is useful for the management of diabetic patients with IAA, though it may be difficult to treat the hyperglycaemia before draining the abscess. In addition, when patients become increasingly hyperglycaemic with increasing insulin demands, systemic infection should be suspected, including intra-abdominal infection and IAA.
Radiological studies
CT scan of the abdomen and pelvis is the preferred imaging modality.[19] CT can determine the size and anatomical location of the IAA.
Contrast enhancement (intravenous and enteral contrast) can help differentiate between intestinal loops and an interloop abdominal abscess, and to determine abscess proximity to vascular structures. This is particularly important in identifying whether a percutaneous approach to drainage of the IAA is possible. Rim enhancement, as well as the presence of debris or contrast leak inside the collection, raises suspicion of the diagnosis of IAA but cannot differentiate completely between an abscess and a sterile fluid collection in the peritoneal cavity. In this circumstance, percutaneous aspiration of the fluid collection and evaluation with Gram stain and culture are recommended to confirm a diagnosis of IAA. The presence of free air inside a fluid collection is diagnostic of an IAA but could also signify connection with the gastrointestinal tract.
Magnetic resonance imaging is useful to show the impact of an abscess on adjacent structures, especially muscle; hence, it is more useful in low pelvic abscesses.[14] Ultrasonography may be a useful aid to the diagnosis, especially when the patient cannot be transported. However, as it is user-dependent, small abscesses may be missed. In addition, ultrasonography does not demonstrate the abscess and its relation to other intra-abdominal viscera. It can also be limited by the presence of a surgical wound, and can be affected by the size of the patient. False positives for IAA in patients with Crohn's disease have also been reported.[23]
Endoscopic ultrasound has been used for evaluating and draining IAA adjacent to the gastrointestinal tract, with the largest experience being with pancreatic fluid collections. Other accessible areas include the pelvis and perirectal space, and the subphrenic and perihepatic spaces. Endoscopic ultrasound requires specialised expertise; data regarding its safety and effectiveness in draining IAA are preliminary. However, endoscopic ultrasound may be useful in critically ill patients requiring bedside procedures or for IAA not amenable to other conventional therapies.[24][Figure caption and citation for the preceding image starts]: Abscess completely replacing pancreas and extending into portal hilum, with multiple gas bubbles and large air/fluid levelFrom the collection of Dr Ali F. Mallat and Dr Lena M. Napolitano; used with permission [Citation ends].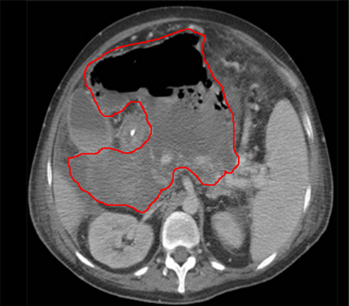 [Figure caption and citation for the preceding image starts]: Intra-abdominal abscess with small air bubble, secondary to perforated diverticulitisFrom the collection of Dr Ali F. Mallat and Dr Lena M. Napolitano; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Intra-abdominal abscess with small air bubble, secondary to perforated diverticulitisFrom the collection of Dr Ali F. Mallat and Dr Lena M. Napolitano; used with permission [Citation ends].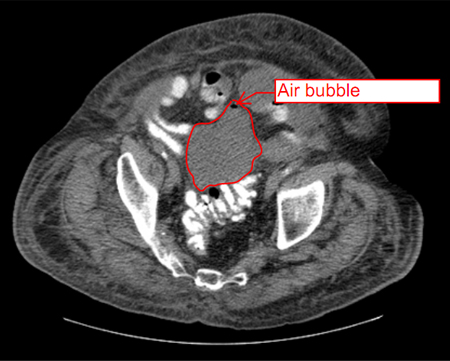 [Figure caption and citation for the preceding image starts]: CT scan showing intra-abdominal abscess with small air bubbleFrom the collection of Dr Ali F. Mallat and Dr Lena M. Napolitano; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: CT scan showing intra-abdominal abscess with small air bubbleFrom the collection of Dr Ali F. Mallat and Dr Lena M. Napolitano; used with permission [Citation ends].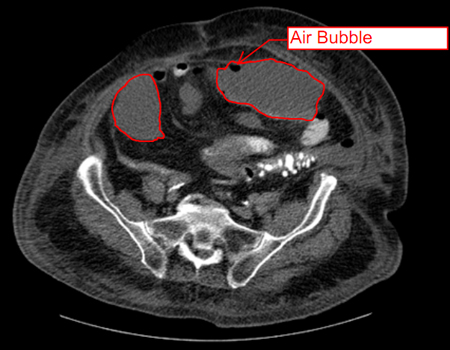
Use of this content is subject to our disclaimer