Approach
Patients with early Dupuytren's contracture can be managed expectantly, although the injection of nodules with a corticosteroid may be carried out if the Dupuytren lesions are bothersome. Those with metacarpophalangeal (MCP) joint contractures of 30 degrees or less and with no proximal interphalangeal (PIP) joint contractures can be treated with needle aponeurotomy, percutaneous fasciotomy, or corticosteroid injections. When function is impaired or a severe and disabling deformity is present, surgery is recommended, and patients with MCP joint contractures >30 degrees or PIP joint contractures can be treated with either open partial fasciectomy, segmental aponeurotomy, or percutaneous fasciotomy.[19][22] Partial fasciectomy has become the favoured technique of hand surgeons in the surgical treatment of Dupuytren's contracture, due to the relatively low rate of recurrence seen with this procedure.[23] It is difficult, however, to compare the efficacy of the many different modalities used to treat Dupuytren's contracture. A reason for this is the inconsistency in reporting of outcomes across studies evaluating treatments for Dupuytren's contracture.[24][25] Only 3 Level I studies have compared the different surgical techniques for the treatment of primary Dupuytren's contracture, and current evidence does not support one procedure as being better than the other, apart from a particularly high recurrence rate after needle fasciotomy. More research will be needed in the future.[26]
All patients with contractures should receive hand therapy post-procedure, and postoperative splinting following open partial fasciectomy is used at the discretion of the surgeon. The effects of stretching on prevention of contracture development for up to 7 months have shown no added benefit, and no studies have been done for more than 7 months. There is therefore no current evidence to support the use of stretching to prevent contractures.[27]
Corticosteroid injections
Patients with early Dupuytren's contracture who have evidence of the disease but have not yet developed contractures can be treated with corticosteroid injections if they are experiencing bothersome symptoms. Those with MCP joint contractures of 30 degrees or less with no PIP joint contractures who wish to avoid a more invasive procedure may also benefit from corticosteroid injections.
The injection of Dupuytren nodules with triamcinolone acetonide monthly for up to 5 months, or every 6 weeks for 3 injections, has been shown to produce significant regression of the disease, with an average of 3.2 injections per nodule required for improvement of function.[28] After corticosteroid injection, fewer patients progress to surgery than would be predicted with expectant management alone.[28]
Collagenase injection
Collagenase clostridium histolyticum is used to treat adult patients with a palpable cord along with MCP or PIP contracture, with a corresponding decrease in both fasciotomies and fasciectomies.[29] The efficacy and tolerability of injectable mixed collagenase subtypes as an alternative to surgical intervention in Dupuytren's contracture has been examined in a phase 3, double-blind study, which found that collagenase safely and effectively restored normal finger extension in the majority (87%) of patients.[30] A mean of 1.4 injections was required to normalise affected joints, and clinical success was achieved within 29 days. Contracture recurrence was relatively low, occurring in just 5 joints (1 MCP, 4 PIP) between 6 and 24 months after treatment, and the recurrence severity ranged from 20 degrees to 40 degrees. Adverse events were localised to the injection site, generally of mild-to-moderate severity, and transient in nature. In another study, more cords that were injected with collagenase met criteria of a reduction in contracture to 0 to 5 degrees of full extension 30 days after the last injection (64.0% vs. 6.8%, P <0.001) than cords injected with placebo.[31] The most commonly reported adverse events were localised swelling in the hand, pain, bruising, pruritus, and transient regional lymph-node enlargement and tenderness. Serious adverse events were seen in 2% of collagenase recipients, including tendon ruptures and complex regional pain syndrome.[31] Although there is some literature with small numbers of patients to suggest that recurrence is more common in PIP joint contractures than in MCP joint contractures, some guidelines recommend that collagenase treatment is limited only to clinical trials.[32][33]
One systematic review has found that having previous surgery did not affect the efficacy and safety of collagenase injections, making this an option in patients with recurrent Dupuytren's contracture.[34]
The technique for collagenase injection is relatively straightforward. First, the skin is prepared with antiseptic solution. Without the use of any local anaesthesia, an insulin needle (28-gauge) is inserted to a depth of 5 mm for MCP joint lesions and 3 mm for PIP joint lesions. The gristly structure of the cord is easily palpated with the end of the needle. A gentle amount of passive motion ensures that the needle is not in the flexor tendon before the injection of collagenase into the MCP or PIP lesion. The injections are administered proximally-to-distally, repositioning the needle prior to injection of the next dose. After the injection is given, a dressing is placed on the hand. The patient is then instructed to return to the surgery the next day for the finger extension manoeuvre, in which the finger is extended to break the cord. This may be done with or without a local anaesthetic. After the manipulation is successfully completed, there are no restrictions and early movement is encouraged. Although wearing a night-time splint afterwards has not been proven to decrease recurrence, it should be offered to the patient. The patient is followed to assess the need for up to 2 further injections every 4 to 6 weeks.[30] Collagenase clostridium histolyticum may not be available in some countries.
Needle aponeurotomy
Needle aponeurotomy, also called percutaneous needle fasciotomy (PNF), is a minimally invasive technique that can be undertaken in the office. While needle aponeurotomy is typically used for early Dupuytren's contracture, it has also been described for more advanced stages of the disease and can be used in those with MCP joint contractures of 30 degrees or less with no PIP joint contractures.
Needle aponeurotomy is usually successful in correcting the Dupuytren's contracture, takes very little time to perform (usually 20 to 30 minutes), requires only local anaesthesia, and is not very painful. In comparison with open surgical procedures, it results in similar resolution,[35][36] minimal scarring, faster recovery, and can be repeated easily if the contracture recurs.[37] Needle aponeurotomy is thus an attractive option for patients with less aggressive and early disease.[38] One systematic review reported a tendency to greater patient satisfaction with needle aponeurotomy, with fewer adverse effects, compared with other procedures.[39] Recurrence rates of up to 58% have been reported during 3 to 5 years of follow-up, but long-term outcomes are not well reported.[40]
Randomised studies report no significant difference in treatment outcome between needle aponeurotomy and collagenase injection.[41][42] In one trial, however, collagenase treatment led to more, mainly transient, complications than needle aponeurotomy.[42]
Percutaneous fasciotomy
Percutaneous fasciotomy is a similar procedure to needle aponeurotomy but uses a scalpel to cut and release the band causing the digital contracture instead of a needle to weaken it.[43][44] It is thus performed by a hand surgeon in the operating room. This technique is normally used in patients with MCP joint contractures of 30 degrees or less with no PIP joint contractures, but can also be used in more advanced stages of Dupuytren's contracture.[22]
As with needle aponeurotomy, the finger is brought into full extension with a characteristic snap, and the goal of percutaneous fasciotomy is to promote greater extension and function of the affected digit.[22]
The major advantages of percutaneous fasciotomy are that it causes less pain and allows a faster recovery than traditional open fasciectomy interventions. However, as with needle aponeurotomy, this procedure is associated with a risk of recurrence of up to 43%, as diseased fascia is unavoidably left behind. There is concomitant risk of flexor tendon or nerve injury.[43][44][45]
Segmental aponeurotomy
Segmental aponeurotomy is a compromise between percutaneous techniques and open fasciectomy, in which multiple small incisions are made in the palm and the digits to remove segments of the Dupuytren cord and achieve discontinuity between the segments of diseased tissue, with no effort made to remove all of the pathological tissue. It is typically used in patients with MCP joint contractures >30 degrees, or in those with PIP joint contractures. The clinical results of this technique compare fairly well with traditional open fasciectomy techniques, with a recurrence rate ranging from 20% to 35%.[46][47][48]
Open partial fasciectomy
Although the timing of surgical intervention varies, the decision to operate is generally taken if the MCP joint contracture exceeds 30 degrees or a PIP joint contracture develops.[49] The presence of any PIP joint contracture alone is a relative indication for surgery.[50] Open partial fasciectomy is a successful treatment and is associated with a postoperative recurrence rate of 15%.[49] The risk of postoperative recurrence increases with severity of the contracture, and the longer a deformity is present, the greater the chance of the joint contracture becoming irreversible.[49][50]
Partial fasciectomy, also known as subtotal, limited, or regional fasciectomy, was first described by Goyrand in 1834 and involves opening up the hand and, under direct visualisation, excising the diseased Dupuytren tissue. Excising only the pathological cord(s) with a partial fasciectomy is now more commonly employed than total fasciectomy or dermatofasciectomy. Postoperative infection is countered with the use of perioperative antibiotics and careful soft tissue handling.
An open partial fasciectomy remains the most common procedure used in the surgical management of Dupuytren's contracture and is the preferred surgical procedure for advanced disease in patients with functional impairment who are surgical candidates. Using this technique, a small amount of potentially diseased fascia could theoretically be left behind, as a radical fasciectomy of the entire palmar fascia is not typically performed. Through a transverse palmar incision, usually overlying the distal palmar crease, the fascia that has formed pathological cords is excised in a proximal-to-distal direction. [Figure caption and citation for the preceding image starts]: Preoperative view of the ring finger of a patient with a flexion contracture with surgical indications, showing the incision marking, demonstrating a transverse incision overlying the distal palmar crease, and oblique Brunner incisions coursing from it proximally and distallyFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].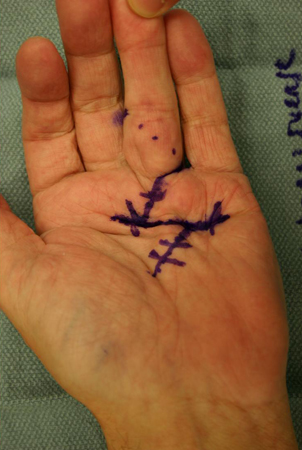 The choice of digital incision is surgeon dependent and is most often between a Brunner incision (angled 'zigzag' incisions made from the ulnar aspect of the MCP joint crease to the radial aspect of the PIP joint or vice versa) and a longitudinal incision (often combined with multiple Z-plasties). Brunner or longitudinal incisions are carried out along the middle of each affected digit, and skin flaps are carefully raised from the pathological cord to avoid skin necrosis. Brunner 'zigzag' incisions are made methodically in a proximal-to-distal direction from the transverse palmar incision, following the path of the palpable Dupuytren cord.
The choice of digital incision is surgeon dependent and is most often between a Brunner incision (angled 'zigzag' incisions made from the ulnar aspect of the MCP joint crease to the radial aspect of the PIP joint or vice versa) and a longitudinal incision (often combined with multiple Z-plasties). Brunner or longitudinal incisions are carried out along the middle of each affected digit, and skin flaps are carefully raised from the pathological cord to avoid skin necrosis. Brunner 'zigzag' incisions are made methodically in a proximal-to-distal direction from the transverse palmar incision, following the path of the palpable Dupuytren cord.
In the palm the neurovascular structures that lie deep to the involved fascia at this level are identified and, with particular attention paid to retracting these neurovascular bundles, the diseased fascia is excised and elevated in a proximal-to-distal direction. [Figure caption and citation for the preceding image starts]: Intraoperative view of the ring finger of a patient with a flexion contracture, with the radial digital neurovascular bundle identified and isolated coursing volar over the Dupuytren cord, which is being held up by forceps as it is excised in a proximal-to-distal directionFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].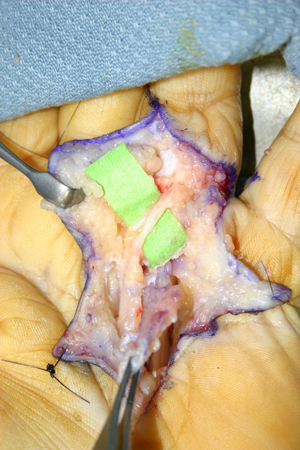 As the Dupuytren cord moves into the finger, great care is taken to identify the radial and ulnar digital arteries and nerves, as they bifurcate from the parent common artery and nerve. As the proper digital neurovascular bundles may be displaced towards the midline in PIP joint contractures, careful dissection is required to identify them in such cases. The fascia must be cut only if it is directly visualised and the artery and nerve are protected. If a spiral cord is present, which is often the case with PIP joint contractures, dissection proceeds from both a proximal-to-distal and a distal-to-proximal direction so as to safely free the neurovascular bundle from the cord that is displacing it centrally.[51] When the small finger is involved, the insertion of the abductor digiti quinti muscle should be identified and excised in order to fully release the digit.
As the Dupuytren cord moves into the finger, great care is taken to identify the radial and ulnar digital arteries and nerves, as they bifurcate from the parent common artery and nerve. As the proper digital neurovascular bundles may be displaced towards the midline in PIP joint contractures, careful dissection is required to identify them in such cases. The fascia must be cut only if it is directly visualised and the artery and nerve are protected. If a spiral cord is present, which is often the case with PIP joint contractures, dissection proceeds from both a proximal-to-distal and a distal-to-proximal direction so as to safely free the neurovascular bundle from the cord that is displacing it centrally.[51] When the small finger is involved, the insertion of the abductor digiti quinti muscle should be identified and excised in order to fully release the digit.
After all of the diseased tissue is excised, the joints are inspected clinically for the persistence of a contracture. Although the MCP joint contracture usually resolves with the excision of the cord alone, PIP joint contractures often do not. Residual contracture of the PIP joint after the fasciectomy is completed is addressed initially with a release of the volar plate, followed by release of the collateral ligaments if necessary.
Currently, most hand surgeons endeavour to dissect the normal skin away from the underlying diseased tissue.[46] If the palmar skin is adherent to the cord so that it cannot be saved and the defect is small, primary closure is often performed. Such defects may also occur as the result of straightening out a previously contracted digit. As the finger is extended, primary skin coverage in the palm may prove to be difficult. If the palmar defect is too large for primary closure, skin grafting or the McCash 'open palm' technique have both shown good clinical results.[52] When possible, direct primary closure of the palmar skin is performed over a Penrose drain to prevent haematoma, as this method of closure allows for early motion and good skin sensibility, avoiding the meticulous wound care required with an open wound. [Figure caption and citation for the preceding image starts]: Postoperative view of the ring finger of a patient with a flexion contracture, showing the closed wound over a Penrose drain, which is used to minimise subsequent haematoma formationFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].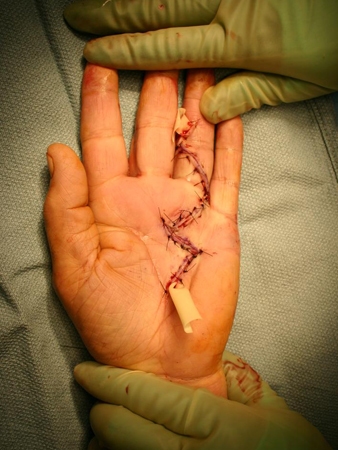 The McCash 'open palm' technique, used by many surgeons, is an alternative to direct palmar wound closure and is associated with a greater active range of motion without any increased risk of infection, although this technique has the disadvantage of leaving the wound open for about 1 month.[52]
The McCash 'open palm' technique, used by many surgeons, is an alternative to direct palmar wound closure and is associated with a greater active range of motion without any increased risk of infection, although this technique has the disadvantage of leaving the wound open for about 1 month.[52]
The fingers are then splinted in full extension, and the patient is followed up within a few days to pull out the drain and assess the wound. By the fifth postoperative day, patients are sent to the hand therapist for a forearm-based digital extension splint that is worn full time between therapy visits. Flexion exercises begin after the wound has stabilised. Regaining digital flexion often proves more difficult than maintaining extension after fasciectomy, due to the postoperative extension splinting required. [Figure caption and citation for the preceding image starts]: One-month postoperative view of the ring finger of a patient with a flexion contracture, demonstrating full active digital extensionFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].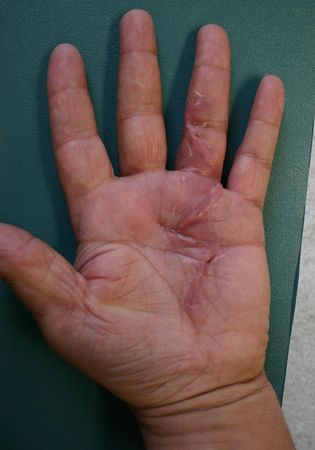 [Figure caption and citation for the preceding image starts]: One-month postoperative view of the ring finger of a patient with a flexion contracture, demonstrating active digital flexionFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: One-month postoperative view of the ring finger of a patient with a flexion contracture, demonstrating active digital flexionFrom the collection of Dr C.M. Rodner; used with permission [Citation ends]. By the third postoperative week, the splint is weaned, to be worn at night only, and night-time extension splinting can continue for as long as 6 months. Some surgeons have abandoned postoperative splinting, favouring earlier mobilisation in order to minimise difficulties with flexion. There is also evidence to suggest that splinting (including night-time extension splinting) after surgery provides no additional benefit to standard hand therapy in maintaining finger extension, except perhaps for cases in which extension loss occurs postoperatively, whereby night-time extension splinting may provide some benefit.[53][54] Postoperative splinting may not be justified in all patients.
By the third postoperative week, the splint is weaned, to be worn at night only, and night-time extension splinting can continue for as long as 6 months. Some surgeons have abandoned postoperative splinting, favouring earlier mobilisation in order to minimise difficulties with flexion. There is also evidence to suggest that splinting (including night-time extension splinting) after surgery provides no additional benefit to standard hand therapy in maintaining finger extension, except perhaps for cases in which extension loss occurs postoperatively, whereby night-time extension splinting may provide some benefit.[53][54] Postoperative splinting may not be justified in all patients.
Other surgical techniques
Total fasciectomy and dermatofasciectomy are now seldom used but are described here for completeness.
The technique of total (or radical) fasciectomy via a transverse palmar incision and multiple digital Z-plasties in which all of the palmar fascia is excised has not been found to have any clinical advantage over a more limited fasciectomy and is associated with a higher complication rate.[55][56] Although total fasciectomy should theoretically reduce recurrence rates by excising the entire palmar fascia responsible for Dupuytren's contracture, this has not been supported by the literature.
Dermatofasciectomy, involving a simultaneous excision of the skin, which is subsequently replaced with full-thickness skin grafts, has produced fairly good results, although morbidity associated with skin grafting, such as poor graft sensibility, has made this technique less popular than partial fasciectomy.[57][58][59][60]
Radiotherapy
Although its use in Dupuytren's contracture is extremely limited, radiotherapy is described here for completeness, as it has been reported to be successful in the treatment of the disease. One study showed that the majority (77%) of lesions did not progress following a total radiotherapy dose of 30 Gy. Long-term studies have not found differences in disease progression 7 years after radiotherapy, compared with controls.[61]
Use of this content is subject to our disclaimer