Approach
Lymphocutaneous/cutaneous sporotrichosis causes skin lesions with characteristic proximal nodular lymphangitic spread.[1][2][13] Because cutaneous nocardiosis, cutaneous non-tuberculous mycobacterial infections, and cutaneous leishmaniasis manifest a similar 'sporotrichoid' skin lesion pattern, the diagnosis of sporotrichosis needs to be established by culture and/or histopathological demonstration of the fungus from aspirated or biopsied skin lesions.
Extracutaneous manifestations of sporotrichosis are more challenging to diagnose because of their non-specific presentation, indolent subacute clinical course, and the lower sensitivity of fungal culture and histopathology in these clinical forms of sporotrichosis. Hence, diagnosis of extracutaneous sporotrichosis is often delayed with reported median times from symptom onset to diagnosis ranging from several months to up to even 2 years depending on the clinical form of sporotrichosis.[Figure caption and citation for the preceding image starts]: Ascending 'sporotrichoid' distribution of skin lesions across the proximal lymphatic channelsFrom the collection of Richard J. Hamill, MD and Edward Septimus, MD, Baylor College of Medicine, Houston, TX [Citation ends].
Lymphocutaneous/cutaneous sporotrichosis
The clinical presentation of characteristic skin lesions with a nodular lymphangitic spread pattern should raise suspicion for sporotrichosis, especially in patients who report trauma during typical occupational and avocational exposures.[1][2][13] The primary skin lesion develops 1 to 12 weeks post fungal inoculation, is non-tender, and usually ulcerates. It is followed by proximal nodular lesions across the lymphatic channels. Most lesions occur in exposed skin of upper extremities and face (lesions on the face are more common in children than in adults). The patients are typically asymptomatic and routine laboratory testing is normal.
The definitive test for diagnosis is fungal culture. Material can be swabbed or aspirated from a skin lesion or a lesion biopsy can be performed. The microbiology laboratory should be notified to keep the Sabouraud's agar cultures for 4 weeks (in 89% of cases, growth occurs within 8 days, but in the remaining 11% of cases growth may take up to 4 weeks).[11] Growth of the Sporothrix schenckii mould form occurs at room temperature; subsequent conversion of the mould to the yeast phase verifies Sporothrix identification.
Histopathological examination of skin lesions using special stains (Gomori-methenamine silver, periodic-acid Schiff) can also be used for diagnosis but has a lower sensitivity compared with fungal culture.[1][2] A mixed pyogenic and granulomatous inflammatory process in the mid-upper dermis with epidermal hyperplasia, hyperkeratosis, and papillomatous acanthosis is typically seen. These inflammatory changes may be less pronounced in patients with HIV. Demonstration of the 3- to 5-micrometre oval- or cigar-shaped yeast forms of S schenckii may be difficult unless multiple skin sections are examined, due to the small number of organisms in tissue sections of patients without HIV infection. This is in contrast to what is seen in patients with HIV, in which large numbers of S schenckii organisms may be present in tissue sections yielding a higher sensitivity for histopathological identification.
The asteroid body may be seen in 20% to 40% of cases in skin biopsy sections. It consists of extracellular eosinophilic material surrounding yeast cells (Splendore-Hoeppli phenomenon). It is more common in sporotrichosis cases from Japan and South Africa than from the Americas, and in lymphocutaneous sporotrichosis compared with all other forms of the infection.
Both microbiological and histopathological examinations of skin lesions are also important for ruling out other infections that manifest with 'sporotrichoid' skin lesion patterns.[31] Routine, acid-fast, and modified acid-fast cultures and stains are necessary for this purpose.
To increase the yield of histopathological examination for diagnosis of lymphocutaneous/cutaneous sporotrichosis, immunohistochemical staining has also been employed, although this method is neither commercially available nor standardised.[32]
Polymerase chain reaction (PCR) using the 18S ribosomal RNA target was reported to be sensitive and specific for diagnosis of cutaneous sporotrichosis in a small number of patients. More clinical studies are required to better define the role of PCR in diagnosis of cutaneous sporotrichosis.[33][Figure caption and citation for the preceding image starts]: Ulcerated primary sporotrichosis lesionFrom the collection of Richard J. Hamill, MD, Baylor College of Medicine, Houston, TX [Citation ends]. [Figure caption and citation for the preceding image starts]: Proximal lymphangitic spread of primary lesionFrom the collection of Richard J. Hamill, MD, Baylor College of Medicine, Houston, TX [Citation ends].
[Figure caption and citation for the preceding image starts]: Proximal lymphangitic spread of primary lesionFrom the collection of Richard J. Hamill, MD, Baylor College of Medicine, Houston, TX [Citation ends].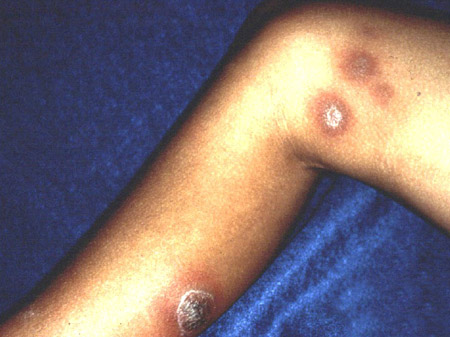 [Figure caption and citation for the preceding image starts]: Cigar- and oval-like yeast form of Sporothrix schenckii typically found in vivo in patient tissues at 37°C (98.6°F)From the collection of Dr Mihalis Lionakis and Dr John E. Bennett [Citation ends].
[Figure caption and citation for the preceding image starts]: Cigar- and oval-like yeast form of Sporothrix schenckii typically found in vivo in patient tissues at 37°C (98.6°F)From the collection of Dr Mihalis Lionakis and Dr John E. Bennett [Citation ends].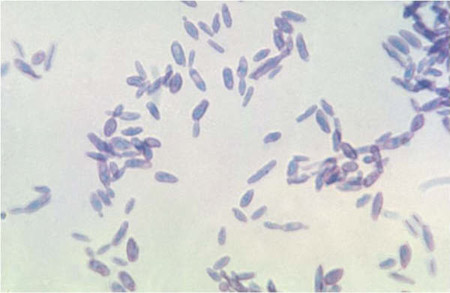 [Figure caption and citation for the preceding image starts]: Mould form of Sporothrix schenckii exhibiting conidia arranged in a characteristic 'bouquet-like' appearance along delicate hyphal structures, typically found in the environment at 25°C to 30°C (77°F to 86°F)From the collection of Dr Mihalis Lionakis and Dr John E. Bennett [Citation ends].
[Figure caption and citation for the preceding image starts]: Mould form of Sporothrix schenckii exhibiting conidia arranged in a characteristic 'bouquet-like' appearance along delicate hyphal structures, typically found in the environment at 25°C to 30°C (77°F to 86°F)From the collection of Dr Mihalis Lionakis and Dr John E. Bennett [Citation ends].
Osteoarticular sporotrichosis
Diagnosis of osteoarticular sporotrichosis is difficult owing to the low yield of microbiological and histopathological methods for diagnosis.[1][2][4][5] Septic arthritis is more common than osteomyelitis. The knee is the joint most frequently affected, followed by the wrist, hand, elbow, and ankle. There is sparing of the shoulder, hip, and spine. Monoarthritis, oligoarthritis, or polyarthritis may occur. Associated bursitis, tenosynovitis, and nerve entrapment syndromes such as carpal tunnel syndrome may develop. Physical examination usually reveals tender swollen joints with accompanying effusions. Nonetheless, unless there are cutaneous sporotrichosis lesions, the diagnosis of osteoarticular sporotrichosis is rarely considered, leading to reported delays in diagnosis of up to 25 months.
Arthrocentesis and histopathology are the available diagnostic tests, although they are not sensitive. Hence, repeated cultures from synovial fluid or tissue are usually necessary to establish the diagnosis. Culture from synovial tissue has a higher yield than culture of synovial fluid. Histopathological demonstration of Sporothrix yeast forms from synovial tissue may be difficult owing to the small number of organisms. Laboratory tests are typically normal except for erythrocyte sedimentation rate elevation.
Imaging is not specific but MRI may show osteomyelitis of bone adjacent to an infected joint. Findings most often seen on routine x-ray include osteoporosis, soft tissue swelling, periosteal reaction, articular surface erosions, and joint effusions.
Pulmonary sporotrichosis
The clinical presentation of pulmonary sporotrichosis is similar to that of pulmonary tuberculosis with fever, chills, night sweats, weight loss, malaise, cough, shortness of breath, and occasionally, haemoptysis.[2][6][13] Patients are typically men aged 30 to 60 years with concomitant conditions (e.g., COPD, pulmonary tuberculosis, diabetes mellitus) and/or alcoholism.[1][2] Repeated cultures from sputum, bronchoalveolar lavage, or bronchial biopsy material are usually necessary for diagnosis. In addition, histopathological demonstration of Sporothrix from bronchial biopsies may reveal the cigar- or oval-shaped Sporothrix yeast forms. Imaging findings are similar to tuberculosis, revealing upper lobe cavitary pneumonia with occasional hilar lymphadenopathy and pleural effusions.
Meningeal sporotrichosis
Meningeal sporotrichosis follows a chronic indolent clinical course, similar to that seen with meningeal histoplasmosis and coccidioidomycosis.[1] Cerebrospinal fluid (CSF) analysis reveals elevated protein, hypoglycorrhachia, and lymphocytic predominance. Repeated cultures of large-volume CSF are necessary to make the diagnosis. Meningeal sporotrichosis should thus be considered in patients with indolent culture-negative meningitis. Computed tomography or magnetic resonance imaging of the brain may occasionally show accompanying enhancing brain lesions. Because of its challenging diagnosis, serological tests have been used. Specifically, an enzyme immunoassay and latex agglutination test of the CSF was reported to be sensitive and specific, although these tests are not commercially available.[34]
Disseminated sporotrichosis
A combination of cutaneous and extracutaneous manifestations occurs in patients with disseminated sporotrichosis.[1][8][9] Chronic meningitis is often diagnosed from concomitant skin lesions because CSF cultures are often negative.[35]
Fungal blood cultures are occasionally positive. Diagnosis is based on culture and/or histopathological demonstration of the fungus from affected tissues such as skin, synovial fluid/tissue, sputum, or CSF.
Emerging tests
PCR has been reported to have high sensitivity and specificity in diagnosis of cutaneous sporotrichosis in one study with 12 patients; no literature exists regarding the role of PCR for diagnosis of extracutaneous manifestations of sporotrichosis. The 18S ribosomal RNA was the reported target gene for the PCR reaction.[33] There is no literature on the utility of beta-glucan in diagnosing sporotrichosis.
Use of this content is subject to our disclaimer