Coeliac disease can present in many varied ways and requires a high degree of clinical suspicion.
Presenting features
Patients with unexplained gastrointestinal symptoms (including those diagnosed with irritable bowel syndrome and/or dyspepsia), chronic diarrhoea, unexplained iron deficiency anaemia, or a skin rash consistent with dermatitis herpetiformis should be tested for coeliac disease.[64]Ford AC, Ching E, Moayyedi P. Meta-analysis: yield of diagnostic tests for coeliac disease in dyspepsia. Aliment Pharmacol Ther. 2009 Jul;30(1):28-36.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2036.2009.04008.x
http://www.ncbi.nlm.nih.gov/pubmed/19416130?tool=bestpractice.com
[65]Ford AC, Chey WD, Talley NJ, et al. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009 Apr 13;169(7):651-8.
http://www.ncbi.nlm.nih.gov/pubmed/19364994?tool=bestpractice.com
[66]Irvine AJ, Chey WD, Ford AC. Screening for celiac disease in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2017 Jan;112(1):65-76.
http://eprints.whiterose.ac.uk/106483
http://www.ncbi.nlm.nih.gov/pubmed/27753436?tool=bestpractice.com
Other situations that may prompt testing include failure to thrive, short stature, vitamin deficiency (B12, D, or folate), recurrent severe aphthous stomatitis, recurrent spontaneous abortion, and infertility.[67]Pastore L, Carroccio A, Compilato D, et al. Oral manifestations of celiac disease. J Clin Gastroenterol. 2008 Mar;42(3):224-32.
http://www.ncbi.nlm.nih.gov/pubmed/18223505?tool=bestpractice.com
Investigations
Before testing, it is crucial to ensure that the patient is ingesting gluten, because all diagnostic tests will usually normalise on a gluten-free diet.
1. Serology
Immunoglobulin A-tissue transglutaminase (IgA-tTG) titre should be evaluated.[68]van der Windt DA, Jellema P, Mulder CJ, et al. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA. 2010 May 5;303(17):1738-46.
http://jama.jamanetwork.com/article.aspx?articleid=185775
http://www.ncbi.nlm.nih.gov/pubmed/20442390?tool=bestpractice.com
[69]Lewis NR, Scott BB. Meta-analysis: deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment Pharmacol Ther. 2010 Jan;31(1):73-81.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2036.2009.04110.x
http://www.ncbi.nlm.nih.gov/pubmed/19664074?tool=bestpractice.com
Quantitative IgA is often routinely requested to assess for IgA deficiency, as the presence of IgA deficiency renders IgA-tTG insensitive. IgA deficiency is more common in people with coeliac disease than in the general population.[70]Chow MA, Lebwohl B, Reilly NR, et al. Immunoglobulin A deficiency in celiac disease. J Clin Gastroenterol. 2012 Nov-Dec;46(10):850-4.
http://www.ncbi.nlm.nih.gov/pubmed/22476042?tool=bestpractice.com
Endomysial antibody (EMA) is a more expensive alternative to IgA-tTG, with greater specificity but lower sensitivity, which may be used if IgA-tTG is unavailable.[71]Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests). Aliment Pharmacol Ther. 2006 Jul 1;24(1):47-54.
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2036.2006.02967.x/full
http://www.ncbi.nlm.nih.gov/pubmed/16803602?tool=bestpractice.com
Unlike tTG, which is an enzyme-linked immunosorbent assay, EMA is based on immunofluorescence and thus is operator dependent.
In patients with IgA deficiency, request IgG-deamidated gliadin peptide (DGP) serology, although the diagnostic accuracy of this test is somewhat less than that of IgA-tTG.[69]Lewis NR, Scott BB. Meta-analysis: deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment Pharmacol Ther. 2010 Jan;31(1):73-81.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2036.2009.04110.x
http://www.ncbi.nlm.nih.gov/pubmed/19664074?tool=bestpractice.com
[72]Volta U, Fabbri A, Parisi C, et al. Old and new serological tests for celiac disease screening. Expert Rev Gastroenterol Hepatol. 2010 Feb;4(1):31-5.
http://www.ncbi.nlm.nih.gov/pubmed/20136587?tool=bestpractice.com
DGP serology has largely replaced IgG-tTG serology for IgA-deficient patients.
In children aged <2 years who do not have IgA deficiency, IgA-TTG is the preferred test to confirm coeliac disease diagnosis, whereas in those with IgA deficiency, IgG-DGP or IgG-tTG can be used as confirmatory tests.[73]Chaudrey KH. ACG guideline: diagnosis and management of celiac disease. Am J Gastroenterol. 2023;118(1):23.
http://www.ncbi.nlm.nih.gov/pubmed/36602833?tool=bestpractice.com
[74]Catassi GN, Pulvirenti A, Monachesi C, et al. Diagnostic accuracy of IgA anti-transglutaminase and IgG anti-deamidated gliadin for diagnosis of celiac disease in children under two years of age: a systematic review and meta-analysis. Nutrients. 2021 Dec 21;14(1):7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8746847
http://www.ncbi.nlm.nih.gov/pubmed/35010880?tool=bestpractice.com
Patients with an elevated IgA-tTG level should be advised to remain on a gluten-containing diet and referred for duodenal biopsy.
A normal IgA-tTG and total IgA test result are adequate to exclude a diagnosis in patients with a low clinical index of suspicion for coeliac disease.[73]Chaudrey KH. ACG guideline: diagnosis and management of celiac disease. Am J Gastroenterol. 2023;118(1):23.
http://www.ncbi.nlm.nih.gov/pubmed/36602833?tool=bestpractice.com
In an individual with a high clinical index of suspicion for coeliac disease, it is reasonable to proceed with oesophagogastroduodenoscopy and duodenal biopsy even in the face of normal serologies.[73]Chaudrey KH. ACG guideline: diagnosis and management of celiac disease. Am J Gastroenterol. 2023;118(1):23.
http://www.ncbi.nlm.nih.gov/pubmed/36602833?tool=bestpractice.com
2. Histology
Patients with an elevated IgA-tTG level should be advised to remain on a gluten-containing diet and referred for duodenal biopsy.
Small intestinal biopsies should be obtained regardless of the IgA-tTG result in patients with a high clinical index of suspicion, as 2% of patients with coeliac disease may not have circulating tTG at the time of diagnosis (seronegative coeliac disease).[75]Volta U, Caio G, Boschetti E, et al. Seronegative celiac disease: shedding light on an obscure clinical entity. Dig Liver Dis. 2016 Sep;48(9):1018-22.
http://www.ncbi.nlm.nih.gov/pubmed/27352981?tool=bestpractice.com
Paediatric patients with symptoms consistent with coeliac disease and a high IgA-tTG titre (above 10 times normal range for laboratory) may go on to have confirmatory EMA testing. If EMA is positive, coeliac disease may be diagnosed without a small intestinal biopsy.[76]Husby S, Koletzko S, Korponay-Szabó I, et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020 Jan;70(1):141-56.
https://journals.lww.com/jpgn/Fulltext/2020/01000/European_Society_Paediatric_Gastroenterology,.24.aspx
http://www.ncbi.nlm.nih.gov/pubmed/31568151?tool=bestpractice.com
Some experts advise that adult patients with very high IgA-tTG titres (above 10 times the normal range for laboratory), and positive EMA in a second blood sample, may be diagnosed without duodenal biopsy.[77]Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease - changing utility of serology and histologic measures: expert review. Gastroenterology. 2019 Mar;156(4):885-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6409202
http://www.ncbi.nlm.nih.gov/pubmed/30578783?tool=bestpractice.com
These criteria may be considered an 'after the fact' diagnosis among adults unwilling or unable to undergo duodenal biopsy.[73]Chaudrey KH. ACG guideline: diagnosis and management of celiac disease. Am J Gastroenterol. 2023;118(1):23.
http://www.ncbi.nlm.nih.gov/pubmed/36602833?tool=bestpractice.com
Duodenal biopsy changes in coeliac disease are typically graded by the Marsh-Oberhuber classification, from 0 to 4.[78]Marsh MN. The immunopathology of small intestinal reaction in gluten-sensitivity. Immunol Invest. 1989 Jan-May;18(1-4):509-31.
http://www.ncbi.nlm.nih.gov/pubmed/2786501?tool=bestpractice.com
To diagnose coeliac disease, intra-epithelial lymphocytes should be increased and the villous-to-crypt ratio decreased. The presence of only one of these changes raises the possibility of a different diagnosis.[Figure caption and citation for the preceding image starts]: Histological image of small intestinal villous atrophy and crypt hyperplasiaFrom the personal collection of DA Leffler; used with permission [Citation ends].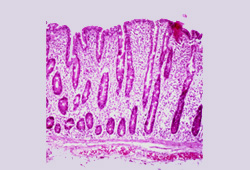
The presence of typical coeliac changes on duodenal histology with clinical improvement on a gluten-free diet confirms the diagnosis. A repeat duodenal biopsy after gluten withdrawal is no longer routinely necessary for verification.[Figure caption and citation for the preceding image starts]: Histological image of small intestinal villi showing resolution of intestinal injury on gluten-free dietFrom the personal collection of DA Leffler; used with permission [Citation ends].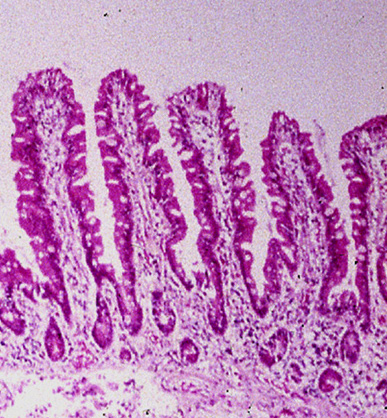 [Figure caption and citation for the preceding image starts]: Photograph of small intestinal villi affected by coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Photograph of small intestinal villi affected by coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].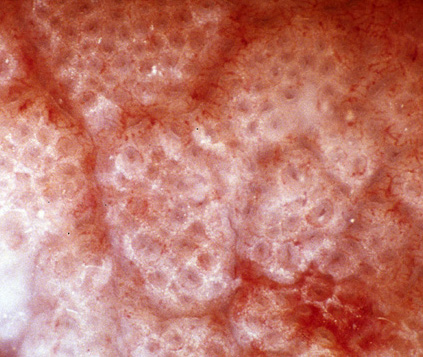 [Figure caption and citation for the preceding image starts]: Photograph of normal small intestinal villiFrom the personal collection of DA Leffler; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Photograph of normal small intestinal villiFrom the personal collection of DA Leffler; used with permission [Citation ends].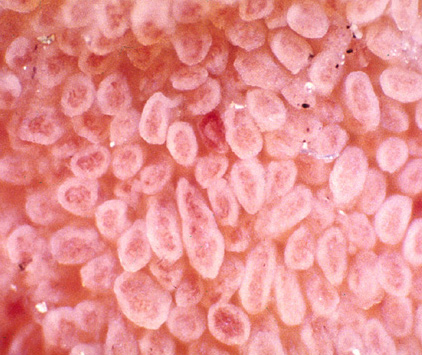 [Figure caption and citation for the preceding image starts]: Capsule endoscopy pictures of ulcerative jejunitis in a patient with coeliac diseaseFrom the personal collection of Amelie Therrien; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Capsule endoscopy pictures of ulcerative jejunitis in a patient with coeliac diseaseFrom the personal collection of Amelie Therrien; used with permission [Citation ends].
3. Human leukocyte antigen (HLA) typing
May be used to rule out coeliac disease in patients already on a gluten-free diet or in patients with an idiopathic coeliac-like enteropathy, but is only helpful for diagnosis in select cases, such as when there is discrepancy between serological and histological findings.[73]Chaudrey KH. ACG guideline: diagnosis and management of celiac disease. Am J Gastroenterol. 2023;118(1):23.
http://www.ncbi.nlm.nih.gov/pubmed/36602833?tool=bestpractice.com
HLA typing may be used as a first-line screening test to rule out coeliac disease among first-degree relatives.[34]Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019 Jun;7(5):583-613.
https://journals.sagepub.com/doi/10.1177/2050640619844125?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed
http://www.ncbi.nlm.nih.gov/pubmed/31210940?tool=bestpractice.com
However, the availability and cost of this test in this context may be prohibitive.
4. Endoscopy
Atrophy and scalloping of mucosal folds; nodularity and mosaic pattern of mucosa may be seen, but these findings are neither sensitive nor specific for coeliac disease diagnosis.[Figure caption and citation for the preceding image starts]: Scalloping of the duodenal mucosa in a patient with coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].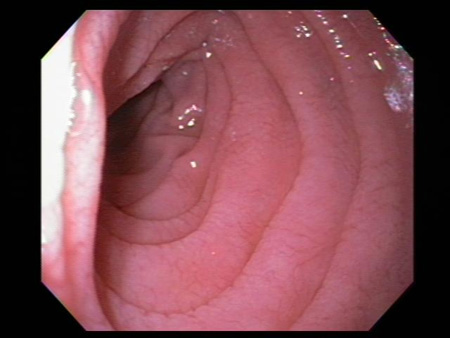 [Figure caption and citation for the preceding image starts]: Scalloping of the duodenal mucosa in a patient with coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Scalloping of the duodenal mucosa in a patient with coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].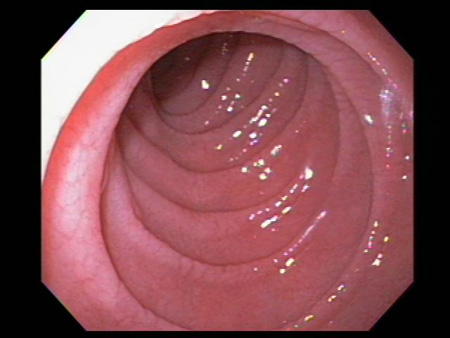
Video capsule endoscopy enables imaging of the entire small intestine and has good sensitivity for the detection of macroscopic features of coeliac disease. Capsule endoscopy is, however, typically used to detect complications of coeliac disease, such as ulcerative jejunitis or lymphoma.[79]Luján-Sanchis M, Pérez-Cuadrado-Robles E, García-Lledó J, et al. Role of capsule endoscopy in suspected celiac disease: a European multi-centre study. World J Gastroenterol. 2017 Jan 28;23(4):703-11.
https://www.wjgnet.com/1007-9327/full/v23/i4/703.htm
http://www.ncbi.nlm.nih.gov/pubmed/28216978?tool=bestpractice.com
[80]Elli L, Casazza G, Locatelli M, et al. Use of enteroscopy for the detection of malignant and premalignant lesions of the small bowel in complicated celiac disease: a meta-analysis. Gastrointest Endosc. 2017 Aug;86(2):264-73;e1.
http://www.ncbi.nlm.nih.gov/pubmed/28433612?tool=bestpractice.com
Gluten challenge
People with coeliac disease on a gluten-free diet prior to evaluation cannot be differentiated from healthy controls. In these patients, gluten challenge is necessary. In a gluten challenge, the person is placed back on a gluten-containing diet, containing 3-10 grams of gluten per day (2-5 slices of wheat bread), with serological tests and small bowel histology assessed after 2-8 weeks on the gluten-containing diet.[81]Leffler DA, Schuppan D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013 Jul;62(7):996-1004.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3525791
http://www.ncbi.nlm.nih.gov/pubmed/22619366?tool=bestpractice.com
[82]Leonard MM, Silvester JA, Leffler D, et al. Evaluating responses to gluten challenge: a randomized, double-blind, 2-dose gluten challenge trial. Gastroenterology. 2021 Feb;160(3):720-33.e8.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/33130104
http://www.ncbi.nlm.nih.gov/pubmed/33130104?tool=bestpractice.com
Commercial kits
Patients who have used a home-testing kit, or are considering using one, should be counselled to discuss their symptoms with their healthcare professional, irrespective of the test outcome.
Commercially available tests for the assessment of individual risk for coeliac disease detect the presence of HLA-DQ2 and HLA-DQ8 genes in saliva.[83]Hearn NL, Chiu CL, Lind JM. Comparison of DNA methylation profiles from saliva in coeliac disease and non-coeliac disease individuals. BMC Med Genomics. 2020 Feb 3;13(1):16.
https://bmcmedgenomics.biomedcentral.com/articles/10.1186/s12920-020-0670-9
http://www.ncbi.nlm.nih.gov/pubmed/32014011?tool=bestpractice.com
However, UK guidelines recommend against HLA-DQ2 and HLA-DQ8 testing in the initial diagnosis of coeliac disease in non‑specialist settings.[84]National Institute for Health and Care Excellence. Coeliac disease: recognition, assessment and management. Sep 2015 [internet publication].
https://www.nice.org.uk/guidance/ng20
Healthcare professionals should be aware that patients who test positive for tTG antibodies using self-administered blood tests (finger-prick tests) may begin a gluten-free diet before being evaluated by their healthcare professional, and this may make subsequent diagnostic workup difficult.[85]Rashid M, Butzner JD, Warren R, et al. Home blood testing for celiac disease: recommendations for management. Can Fam Physician. 2009 Feb;55(2):151-3.
https://www.cfp.ca/content/55/2/151.long
http://www.ncbi.nlm.nih.gov/pubmed/19221072?tool=bestpractice.com
Tests for detection of tTG antibodies in saliva are being investigated, but there is insufficient evidence to recommend their use.[34]Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019 Jun;7(5):583-613.
https://journals.sagepub.com/doi/10.1177/2050640619844125?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed
http://www.ncbi.nlm.nih.gov/pubmed/31210940?tool=bestpractice.com

 [Figure caption and citation for the preceding image starts]: Photograph of small intestinal villi affected by coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Photograph of small intestinal villi affected by coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Photograph of normal small intestinal villiFrom the personal collection of DA Leffler; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Photograph of normal small intestinal villiFrom the personal collection of DA Leffler; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Capsule endoscopy pictures of ulcerative jejunitis in a patient with coeliac diseaseFrom the personal collection of Amelie Therrien; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Capsule endoscopy pictures of ulcerative jejunitis in a patient with coeliac diseaseFrom the personal collection of Amelie Therrien; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Scalloping of the duodenal mucosa in a patient with coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Scalloping of the duodenal mucosa in a patient with coeliac diseaseFrom the personal collection of DA Leffler; used with permission [Citation ends].
