Approach
The approach to diagnosis will depend on the status of the patient.
Pregnant women
Acute infection during pregnancy is often asymptomatic in women, but when transmitted to the fetus can have devastating consequences. Antenatal screening for Toxoplasma is not routinely performed in the US. However, some countries (such as France and Austria) do recommend routine testing during pregnancy.
Pregnant women with new cervical/occipital lymphadenopathy, or evidence of microcephaly, intracranial calcifications, hydrocephalus, or intrauterine growth restriction in the fetus detected by sonogram, raise suspicion for acute or recent infection.
In these cases, anti-Toxoplasma immunoglobulin (Ig) G and IgM should be checked in the mother. A negative anti-Toxoplasma IgM rules out acute infection. In rare cases, testing for IgM may have to be repeated if initially negative and where there is strong clinical suspicion of infection. In areas of high prevalence, serologies may be checked more than once during pregnancy.
If both IgM and IgG are negative, the mother likely has had no exposure to Toxoplasmagondii. If IgG is positive and IgM is negative, the mother has a remote history of infection and there is little risk of infection in the fetus. If anti-Toxoplasma IgM is positive, acute infection cannot be ruled out and more definitive testing from a Toxoplasma reference laboratory is needed. A positive IgM result is not proof of acute infection: IgM may persist for up to 1 year after acute infection and there are high rates of false positives with some testing methods. Toxoplasma-specific IgG avidity index is useful in pregnant women who have detectable IgG and IgM as it can differentiate between recent and chronic infection. Epidemiologically, new infections are the only ones with significant risk to the fetus; however, the mechanism of IgG preventing infection in the fetus is unknown.
Reference labs have the ability to perform a panel of tests, including the dye test for IgG, IgM enzyme-linked immunosorbent assay (ELISA), differential agglutination test, IgA ELISA, and IgE immunosorbent agglutination assay (ISAGA)/ELISA for confirmatory testing.[11] If it is determined that the mother has acute infection, Toxoplasma polymerase chain reaction (PCR) of amniotic fluid should be performed to evaluate for transmission to the fetus.[31] This should be done at ≥4 weeks after acute primary maternal infection and at ≥18 weeks of gestation to reduce the risk of false negative results.[11] Sonogram of the fetus can also be performed to evaluate for ventricular dilatation, intracranial calcifications, ascites, and hepatomegaly.[11]
Newborn of mother with suspected infection during pregnancy
Congenital disease is often subclinical in the newborn and may mimic other neonatal diseases, making its diagnosis more complicated than the diagnosis of acquired infection in adults. In addition, serological diagnosis is more difficult because maternal IgG passes transplacentally to the fetus.
History and physical examination, including paediatric neurological evaluation and ophthalmological examination of the retina, should be performed on all newborns at risk for congenital disease in an attempt to find clinical signs of disease.
Serological evaluation should be performed by an experienced reference laboratory and often includes: quantitative immunoglobulins, dye test for IgG, IgM ISAGA, and IgA ELISA.[11] The presence of Toxoplasma-specific IgG, IgM, and/or IgA antibodies in neonates supports a diagnosis of in utero infection.[11] Consultation with an infectious diseases consultant is recommended for guidance in interpreting serology results and in determining a course of treatment for the newborn.
Toxoplasma PCR assays (peripheral blood, cerebrospinal fluid, and urine) should be performed as soon as possible after birth and if detected confirms the diagnosis.[11] In addition, perform full blood count with differential, liver function tests including gamma-glutamyl transferase and bilirubins, cerebrospinal fluid evaluation for cell count, protein, glucose, quantitative IgG and toxo-specific IgG/IgM, and brain imaging.[11]
Interferon-gamma release assay, which measures interferon-gamma production from whole blood stimulated with T gondii antigen may be used to rule out congenital infection in newborns and thus avoid the need for serological follow-up. The sensitivity and specificity of the test in infants suspected of having congenital disease have been reported as 94% and 98%, respectively.[32] However, the test is not commercially available or routinely performed by reference laboratories. In its absence, congenital toxoplasmosis can be definitively diagnosed by the persistence of Toxoplasma IgG antibodies beyond the first year of life, reflecting the infant's immune response.[11]
Patients who are immunocompromised
Suspicion for toxoplasmosis should be high in a patient who is immunocompromised, whether because of HIV/AIDS, immunosuppressive medications, or other causes, and who presents with fever and a change in mental status, seizure, or other focal neurologic deficit with enhancing lesions on computed tomography or magnetic resonance imaging of the brain. If a patient who is immunosuppressed presents with fever or malaise and hepatitis, pneumonitis or myocarditis, or with chorioretinitis, toxoplasmosis should be included in the differential diagnosis. Central nervous system lesions are usually multiple and are more commonly seen in HIV-infected patients. Transplant recipients may instead present with cardiac, pulmonary, or disseminated disease.
The first test to order in evaluation of toxoplasmosis is serology for anti-Toxoplasma IgG. However, serologies may be difficult to interpret in those who are immunocompromised because of generally low levels of immunoglobulins. Toxoplasma PCR can be done on a blood sample, or on other body fluids or tissues depending on localisation of symptoms. In addition, biopsy of an affected organ (e.g., cardiac biopsy in a patient with myocarditis) may reveal the diagnosis.
Enzyme-linked immunosorbent spot assay may be helpful in stratifying risk of recurrence of toxoplasmic encephalitis in T gondii IgG+ HIV-infected patients.[33] However, it is not used by reference laboratories because cut-off values to discriminate among patients with adequate or insufficient T gondii-specific immune responses have not been determined.[Figure caption and citation for the preceding image starts]: Computed tomography: brain of central nervous system toxoplasmosisFrom the collection of Louis M. Weiss, MD, MPH; used with permission [Citation ends].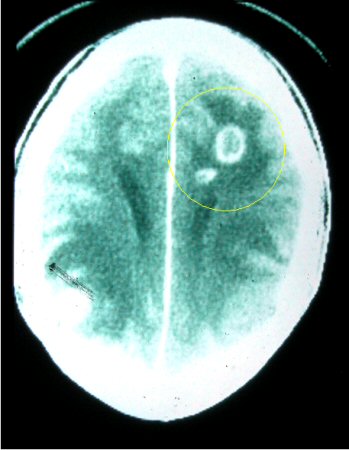 [Figure caption and citation for the preceding image starts]: Brain pathology: while this is pathology from mouse tissue, it is quite similar to the appearance in human tissueFrom the collection of Louis M. Weiss, MD, MPH; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Brain pathology: while this is pathology from mouse tissue, it is quite similar to the appearance in human tissueFrom the collection of Louis M. Weiss, MD, MPH; used with permission [Citation ends].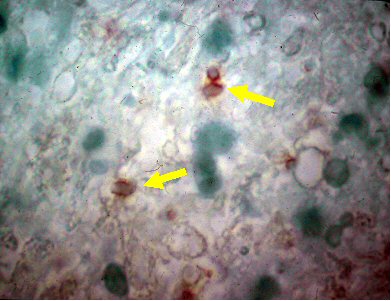
Chorioretinitis
Most cases of toxoplasmic chorioretinitis result from congenital infection that does not become clinically apparent until after re-activation in the eye. However, outbreaks of acute chorioretinitis associated with water contamination have been reported.[34] While the presence of lesions in the fundus should raise suspicion for toxoplasmosis, proof that Toxoplasma is the cause of such disease is often lacking. Serum antibody titres are usually low in the presence of active eye lesions caused by congenital disease. In addition, the appearance of inflammatory lesions is not unique to toxoplasmosis. Similar lesions can be found with other granulomatous diseases such as tuberculosis, cat-scratch disease, or toxocariasis. In people who are immunocompromised, cytomegalovirus, herpes simplex virus, or syphilis must also be considered.
After a full ophthalmological examination reveals evidence of a retinal lesion with associated inflammation, serology should be checked for evidence of prior infection. In general, if the retinal lesions are characteristic and serologies are positive, the diagnosis of toxoplasmic chorioretinitis is probable. If the retinal lesions are atypical and the serologies are positive, diagnosis is less certain. Toxoplasma PCR can be performed on aqueous or vitreous fluid from the eye to aid in diagnosis and is commercially available.[35]
If the patient is a newborn or infant with congenital disease, typical lesions are bilateral. If the patient is a child or adolescent with re-activation of congenital disease, active lesions may appear at the periphery of prior retinal scars, and are usually unilateral. In both cases, anti-Toxoplasma IgG will be present, although probably at low titres, and IgM is usually absent.
The American Academy of Pediatrics recommends serial assessment, preferably by a retinal specialist, for infants with confirmed congenital toxoplasmosis until the end of their third year of life, even if their initial examination is negative. They also recommend close ophthalmological follow up of infants with a high suspicion of congenital toxoplasmosis until infection is ruled out on serology.[11]
If the patient is an adult with acute disease of the eye, active retinal lesions are usually unilateral and IgM and/or IgG will be positive. Evidence suggests gender differences in ocular toxoplasmosis presentations. Men more frequently present with primary active disease compared with women (24.4% vs 12.9%), whereas women more often experience recurrent active disease (36.0% vs. 28.5% in men). Additionally, retinal lesions including the macula are more common in women (56.1% vs. 39.8% for men), indicating a potential increased risk of central vision impairment. Despite these differences in clinical presentations, overall outcomes like visual acuity and ocular complications were similar.[36]
If anti-Toxoplasma IgG and IgM are negative in undiluted serum, chorioretinitis is probably not due to toxoplasmosis. Demonstration of anti-Toxoplasma antibodies or of Toxoplasma DNA by PCR in aqueous humour from the anterior chamber of the affected eye can establish a diagnosis in equivocal cases, but the risks of this procedure often do not outweigh the low risk of a short treatment course, and clinicians often try a course of treatment.[37]
Use of this content is subject to our disclaimer