Approach
Patients with signs and symptoms of syphilis should undergo diagnostic testing.[36] In patients with asymptomatic infection, diagnosis relies on routine screening.
History
Eliciting a history of sexual activity and risk factors is important when considering the diagnosis of syphilis. People at high risk of infection include those who have had sexual contact with an infected person, men who have sex with men (MSM), people infected with HIV or other STIs, people with multiple sexual partners, commercial sex workers, and people using illicit drugs. Pregnant women with syphilis are at risk of transmitting the infection transplacentally to the fetus.
It is important to establish whether a patient has a history of syphilis (and past treatment), as this can help with the interpretation of diagnostic test results and help confirm the stage of infection.
Signs and symptoms of primary syphilis
A solitary painless genital ulcer (chancre) in the anogenital or cervix area strongly suggests a diagnosis of primary syphilis.[37] It may not always be noticed by the patient and examining physician, and it heals spontaneously. There may also be discrete, painless, rubbery regional lymphadenopathy. Mouth ulceration may occur in primary infection. When this occurs, the ulcer is confined to the mouth. [Figure caption and citation for the preceding image starts]: A primary vulvar syphilitic chancre due to Treponema pallidum bacteriaCDC: PHIL image ID 5340; used with permission [Citation ends].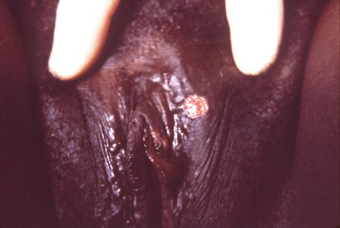 [Figure caption and citation for the preceding image starts]: A penile chancre located on the proximal penile shaft: primary syphilitic infectionCDC/ Dr Gavin Hart; Dr NJ Fiumara; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: A penile chancre located on the proximal penile shaft: primary syphilitic infectionCDC/ Dr Gavin Hart; Dr NJ Fiumara; used with permission [Citation ends].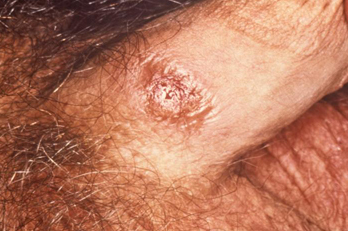
Atypically, ulceration may be multiple and painful. Co-infection with genital herpes or chancroid may be a cause of painful ulceration. Co-infection with HIV may result in multiple ulcers: approximately 30% of HIV antibody-negative and 70% of HIV antibody-positive patients with primary syphilis have multiple genital ulcers.[38]
Signs and symptoms of secondary syphilis
Clinical features of secondary syphilis typically appear 4 to 8 weeks after primary syphilis infection, but they may also occur up to 6 months later.[6] The presentation of secondary syphilis is diverse. Haematogenous dissemination in secondary syphilis affects different organs. Patients may describe non-specific symptoms including fever, malaise, myalgia, fatigue, or arthralgia. They may also notice generalised lymphadenopathy. These features may be mistaken for an intercurrent viral illness or primary HIV infection. There may be a generalised symmetrical macular, papular, or maculopapular diffuse rash, typically affecting the palms of the hands and plantar aspects of the feet. The rash may also occur on the trunk and scalp. Occasionally, the papules may ulcerate. There may be generalised mucosal ulceration, causing 'snail-track' ulcers on the buccal mucosa, and erosions on the genitalia. There may be flesh-coloured wart-like lesions in the genital area, known as condylomata lata. Patchy alopecia may develop.
[Figure caption and citation for the preceding image starts]: Secondary syphilitic papulosquamous rash on the torso and upper bodyCDC/Susan Lindsley; used with permission [Citation ends].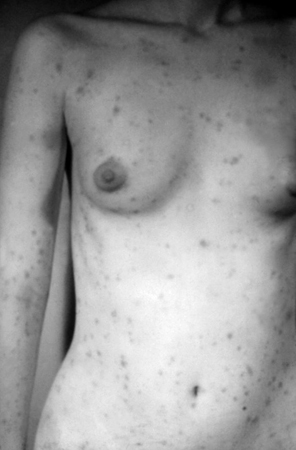 [Figure caption and citation for the preceding image starts]: Secondary syphilitic lesions on the faceCDC: PHIL image ID 3500; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Secondary syphilitic lesions on the faceCDC: PHIL image ID 3500; used with permission [Citation ends].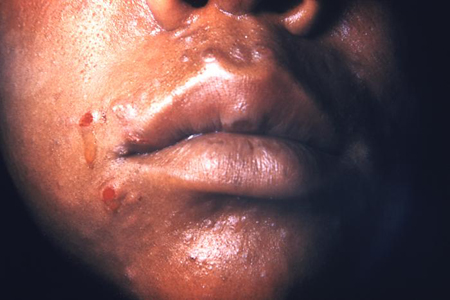 [Figure caption and citation for the preceding image starts]: Secondary syphilis presenting pigmented macules and papules on the skinCDC/Susan Lindsley; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Secondary syphilis presenting pigmented macules and papules on the skinCDC/Susan Lindsley; used with permission [Citation ends].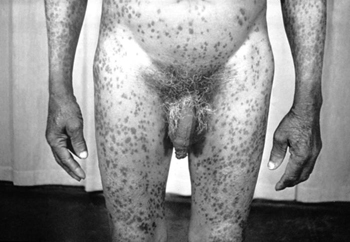 [Figure caption and citation for the preceding image starts]: Secondary syphilitic lesions of vaginaCDC/J. Pledger; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Secondary syphilitic lesions of vaginaCDC/J. Pledger; used with permission [Citation ends].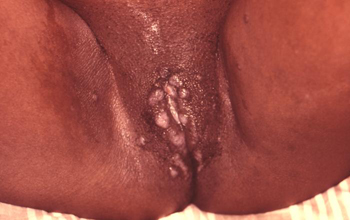
Uncommon presentation includes specific organ involvement. Symptoms of headache, meningismus, hearing loss, seizures, or neuropathy suggest neurological involvement. Neurosyphilis may occur at any stage of infection with syphilis, and may occur in up to 10% of patients with untreated syphilis.[21][39] Visual changes due to syphilitic iritis, uveitis, and chorioretinitis may initially present to ophthalmological services.[40] The vasculitis due to secondary syphilis may cause a nephrotic syndrome, glomerulonephritis, or hepatitis.
Up to 25% of people who have untreated secondary syphilis go on to develop relapsing episodes of secondary syphilis.[9][19] Symptoms include rash and fever. These relapsing episodes rarely occur more than 1 year after acquiring syphilis.
Latent syphilis
Latent syphilis is defined as positive serology in the absence of clinical features of syphilis. Early latent syphilis is defined as asymptomatic infection that is diagnosed on the basis of positive serology alone, acquired <1 year previously (according to the US Centers for Disease Control and Prevention [CDC]) or <2 years previously (according to the World Health Organization [WHO]).[7][8]
Late latent syphilis is defined as asymptomatic infection that is acquired >1 year previously (CDC) or >2 years previously (WHO).[7][8] The patient is not known to have been seronegative within the past year (CDC) or past 2 years (WHO).[7][8]
Signs and symptoms of tertiary syphilis
Tertiary syphilis develops in 14% to 40% of patients with untreated syphilis (late symptomatic disease).[6][20] It is characterised by chronic, end-organ complications, often many years after initial infection. The diagnosis may be suspected from a past history of features of earlier-stage disease and the presence of risk factors.
Neurosyphilis may involve damage to the dorsal columns of the spinal cord, causing a syndrome known as tabes dorsalis. Features of tabes dorsalis include:[20]
Ataxia
Loss of anal and bladder sphincter control
Argyll-Robertson pupils
Areflexia
Dorsal column loss (loss of vibration and proprioception/position sense)
Romberg's sign.
Brain involvement causes a range of syndromes, including cognitive and motor impairment, which are sometimes grouped under the broad term 'general paresis'. Features of general paresis may include:[20]
Behavioural changes
Memory impairment
Altered mood
Confusion
Seizures
Tremor
Argyll-Robertson pupils.
A neurology or psychiatric consultation is required if neurosyphilis or brain involvement is suspected.
Cardiovascular syphilis usually affects the aortic root, causing an aortitis, which results in aortic regurgitation. Angina may arise as a result of coronary ostial stenosis. Aortic medial necrosis may cause aortic aneurysm. The cardiac murmur of aortic regurgitation and/or symptoms and signs of heart failure or aortic aneurysm on clinical examination require a cardiology consultation.
Gummatous syphilis (also known as benign tertiary syphilis) affects skin and visceral organs, causing organomegaly and infiltrative or destructive lesions, as well as perforation or collapse of affected structures. Gumma lesions consist of granulomatous rubbery tissue with a necrotic centre. The destructive lesions may gradually replace normal tissue. Gummata are an extremely rare manifestation of late syphilis, with the most common presentation being chronic skin ulceration and nodular infiltration. [Figure caption and citation for the preceding image starts]: Gummatous lesions on the dorsal surface of the left handCDC/Susan Lindsley; used with permission [Citation ends].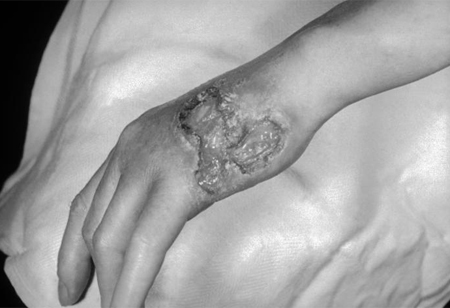
HIV co-infection
Syphilis is an important facilitator of HIV transmission.[19] MSM are at particular risk of co-infection with HIV.[23][41][42] The presence of HIV may alter the presentation of syphilis.[19]
Primary syphilis: larger, painful multiple ulcers.
Secondary syphilis: genital ulcers more common and higher titres with rapid plasma reagin (RPR) testing and Venereal Disease Research Laboratory (VDRL) testing.
Possibly more rapid progression to neurosyphilis.[20]
Serological responses to infection may be atypical.[40]
Signs and symptoms of congenital syphilis
Congenital syphilis occurs when the fetus acquires the infection transplacentally from the mother. This may result in congenital malformations, miscarriage, stillbirth, or neonatal death.[3] Intrauterine features such as hydrops may be detected on fetal ultrasound scanning. Postnatal manifestations are divided into early and late stages; early manifestations occur in the first 2 years of life, and late manifestations occur after 2 years of age.
The diagnosis of congenital syphilis is confirmed or suspected (highly probable, possible, less likely, or unlikely) by considering various factors, including:[8]
Identification of syphilis in the mother
Adequacy of maternal treatment
Presence of clinical, laboratory, or radiographic evidence of syphilis in the infant (testing should include paired maternal and neonatal non-treponemal serological titres using the same test, preferably conducted at the same laboratory).
Most clinical signs are not visible at birth, but usually develop within 3 months. A highly infectious rhinitis, which may be purulent or blood-stained, may persist and is one of the earliest signs. Other early signs (occurring within 2 years) include hepatosplenomegaly, glomerulonephritis and nephrotic syndrome, generalised lymphadenopathy, central nervous system (CNS) involvement (including cerebrospinal fluid [CSF] abnormalities and syphilitic meningitis), and bone involvement (e.g., osteochondritis).[6][19] A neonatal skin rash may occur and may be similar to the rash of secondary syphilis in adults. It may also be more widespread, bullous or papulonecrotic, or desquamating. Initially, the rash may be vesicular with small blisters appearing on the palms and plantar surfaces of the feet. An erythematous or maculopapular rash, which is often copper-coloured, may subsequently appear on the face, palms, and plantar surfaces of the feet. Necrotising funisitis (inflammation of the umbilical cord) is virtually diagnostic of congenital syphilis and is found usually in pre-term infants who are stillborn, or die within a few weeks of birth. The umbilical cord has a specific appearance known as the 'barberpole' cord as a result of inflammation of the matrix of the umbilical cord.[43][Figure caption and citation for the preceding image starts]: This was a case of congenital syphilis resulting in the death of this newborn infantCDC: PHIL image ID 3510; used with permission [Citation ends].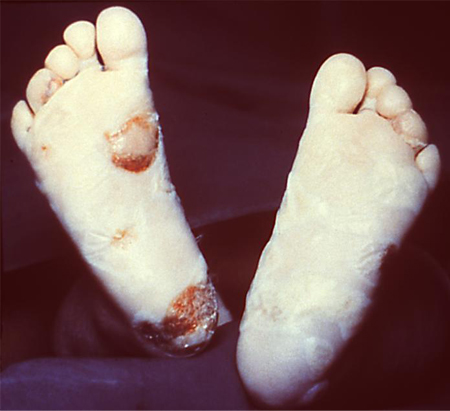 [Figure caption and citation for the preceding image starts]: This newborn presented with symptoms of congenital syphilis that included lesions on the soles of both feetCDC: PHIL image ID 4148; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: This newborn presented with symptoms of congenital syphilis that included lesions on the soles of both feetCDC: PHIL image ID 4148; used with permission [Citation ends].
Untreated congenital syphilis may present late (after age 2 years). It is important to distinguish late congenital syphilis from postnatally acquired syphilis, as the latter raises the suspicion of child sexual abuse and should be investigated further.[44]
Late congenital syphilis has several distinct findings, including:[19][Figure caption and citation for the preceding image starts]: Interstitial keratitisCDC/Susan Lindsley [Citation ends].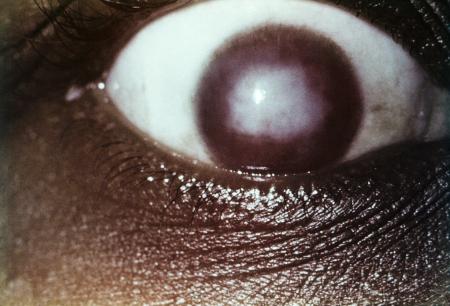 [Figure caption and citation for the preceding image starts]: Clutton's jointsCDC/Richard Deitrick; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Clutton's jointsCDC/Richard Deitrick; used with permission [Citation ends].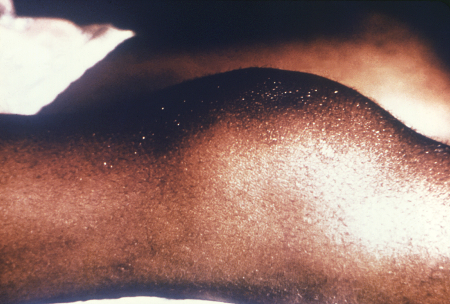 [Figure caption and citation for the preceding image starts]: Peg-shaped, notched central incisors (Hutchinson's teeth)CDC/Robert E. Sumpter; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Peg-shaped, notched central incisors (Hutchinson's teeth)CDC/Robert E. Sumpter; used with permission [Citation ends].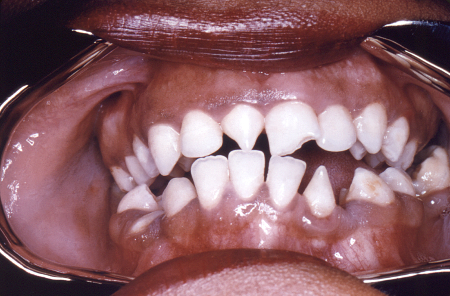 [Figure caption and citation for the preceding image starts]: Osteoperiostitis of the tibia ('saber shins')CDC/Robert E. Sumpter; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Osteoperiostitis of the tibia ('saber shins')CDC/Robert E. Sumpter; used with permission [Citation ends].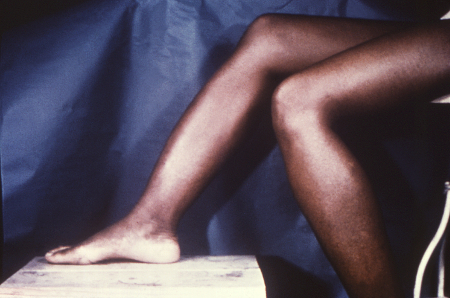
Interstitial keratitis
Peg-shaped central incisors, notched at the apex (Hutchinson's teeth)
Eighth cranial nerve deafness
Frontal bossing of the skull
Anterior bowing of the shins (Saber shins)
Saddle nose deformity
Clutton's joints (symmetric painless knee swelling).
Interstitial keratitis, Hutchinson's teeth, and eighth cranial nerve deafness are collectively known as Hutchinson's triad.
Initial investigations for acquired syphilis
Microscopic tests:
Until 2008, it was not possible to culture Treponema pallidum (T pallidum) in vitro. A complex culture method has been published.[16] Direct detection of T pallidum with dark-field microscopy of the skin lesion can provide a definitive diagnosis of syphilis, but this test is not usually available outside specialist settings.[36] The lesion is cleansed and abraded with a gauze pad until serous exudates appear, which are then collected onto a glass slide for microscopic analysis. Identification of T pallidum from the sample allows for immediate diagnosis. A single negative result does not exclude infection as collection of the treponemes is operator-dependent.[7] Sensitivity of dark-field microscopy for genital ulcers is 74% to 86% and specificity is 85% to 100%.[5][6][45]
For secondary syphilis, dark-field microscopy may be positive from skin or ulcerative anogenital lesions. However, gummata in tertiary syphilis have few, if any, identifiable T pallidum organisms. If available, dark-field microscopy should also be performed on any lesions or nasal discharge in infants with possible congenital syphilis.
Serological tests:
Serology testing is the most commonly used method for diagnosing syphilis. It should be performed in all patients with signs or symptoms of syphilis (e.g., a painless anogenital ulcer). Serology testing using of both non-treponemal (non-specific) and treponemal (specific) tests in combination, if the first test is positive, aids the diagnosis of syphilis infection.[36] The most common approach is to use a treponemal test as the initial serological test, followed by a non-treponemal test if the treponemal test is positive (i.e., a ‘reverse sequence screening algorithm’), but it is also acceptable to use a nontreponemal test as the initial test (i.e., a 'traditional sequence screening algorithm').[36][46] The reverse sequence screening algorithm approach reduces time and costs compared with the traditional sequence screening algorithm approach.[46][47]
Treponemal tests include:[5][48]
Treponemal enzyme immunoassay (EIA)
T pallidum particle agglutination assay (TPPA)
T pallidum haemagglutination assay (TPHA)
Fluorescent antibody absorption (FTA-ABS)
Immunocapture assay (ICA).
Treponemal tests are antigen-based tests and work by detecting antibodies to T pallidum. The TPPA test is the preferred treponemal test.[36] A patient with a positive treponemal test result will remain positive for life, irrespective of current or past infection. Therefore, a positive result alone cannot distinguish between an active infection (i.e., currently untreated or incompletely treated) and a past (treated) infection. Another limitation is that false-positive results may occur in the presence of diseases caused by non-sexually transmitted treponemal infections (e.g., yaws, pinta, bejel). False-negative results may occur in incubating and early primary syphilis.[49]
A non-treponemal test should always be undertaken following a positive treponemal test to confirm a diagnosis and provide evidence of active disease or re-infection. Non-treponemal tests include:[8][25]
RPR test
VDRL test
The RPR test is usually the test of choice due to ease of use and interpretation. Non-treponemal tests work by detecting the antibody response to the release of cardiolipin during syphilis infection. These tests can provide a quantitative measure of disease activity (titre) and can be used to monitor treatment response.[25] RPR and VDRL titres decrease or become non-reactive with effective treatment.[8] Despite adequate treatment, some patients maintain a persisting low level positive antibody titre (known as a serofast reaction).[50] False positives may occur due to the presence of a variety of medical conditions, such as pregnancy, autoimmune disorders, and other infections. A false-negative test may occasionally occur in an undiluted specimen (the prozone phenomenon).
If the non-treponemal test is negative, then a different treponemal test - preferably a TPPA or treponemal assay based on different antigens than the original test - should be performed to confirm the results of the initial treponemal test.[8]
The same non-treponemal test should be used sequentially when monitoring treatment response.[36] This is because results obtained from one test are not directly comparable with that of the next non-treponemal test. A fourfold change in titre, equivalent to a change of two dilutions (e.g., from 1:16 to 1:4 or from 1:8 to 1:32), signifies a clinically significant difference between two non-treponemal test results.[8] Serological tests should be interpreted in the same way for patients with HIV.[36] However, serological responses may be atypical, with higher than expected post-treatment titres (i.e., high serofast) or fluctuating titres. When clinical findings are indicative of syphilis, but serological tests are non-reactive or their interpretation is unclear, alternative diagnostic tests (e.g., biopsy of a lesion, dark-field examination, or polymerase chain reaction [PCR] of lesion material) should be considered.[8]
Incubation periods (usual time after infection that the test becomes positive) for treponemal and non-treponemal tests are as follows.
Treponemal tests
EIA: 3 weeks
TPPA: 4-6 weeks
TPHA: 4-6 weeks.
Non-treponemal tests
RPR: 4 weeks
VDRL: 4 weeks.
Patients with secondary syphilis will have strongly positive syphilis serological tests. Delayed seroreactivity or false-negative non-treponemal serology may rarely occur if there is HIV co-infection.[40][51][52]
Patients with early or late latent syphilis may be detected as part of screening blood tests (e.g., prior to blood donation). EIA is the serological treponemal test generally used for screening.[53] In late latent syphilis, treponemal tests are always positive.
In tertiary syphilis, positive serology will suggest a diagnosis already suspected from the history and clinical signs.
Other initial investigations for acquired syphilis
Line immunoassay (LIA) serological tests (e.g., INNO-LIA Syphilis test) can be used to confirm syphilis infection following initial serological treponemal testing. A single LIA test can confirm infection, making it more convenient than traditional methods of serological confirmation, which usually require performing multiple assays. Studies evaluating the performance of LIA tests for syphilis infection have demonstrated higher sensitivity and specificity compared with FTA-ABS and TPHA serology tests.[54][55]
Emerging investigations
Compared with current tests (e.g., serology, dark-field microscopy), PCR testing for T pallidum using samples taken directly from ulcerative lesions has been found to be moderately sensitive (70% to 80%) and highly specific (>90%) for diagnosing primary and secondary syphilis.[56] The CDC considers PCR testing a valid method for diagnosing primary, secondary, and congenital syphilis, and its use is likely to increase.[8][57]
Point of care (POC) serological testing with either treponemal or combination treponemal/non-treponemal tests has been assessed in the setting of high-risk regions, where rapid and early diagnosis may be more important than accuracy. Several clinical trials have shown promise and POC testing has been recommended as part of the Pan American Health Organization strategy to diagnose and treat syphilis.[58][59]
Further investigations for acquired syphilis
A lumbar puncture and CSF examination should be performed in any patient with clinical evidence of neurosyphilis (e.g., cranial nerve dysfunction, meningitis, stroke, acute or chronic altered mental status, or loss of vibration sense).[8] CNS involvement can occur at any stage of syphilis and can range from asymptomatic meningeal involvement to dementia and sensory neuropathy. A computed tomography or magnetic resonance imaging brain scan should be performed first if there is concern regarding raised intracranial pressure (i.e., mainly to ensure that undertaking a lumbar puncture is safe). A lumbar puncture is also indicated if syphilis of unknown duration is diagnosed in the presence of HIV co-infection. Neurosyphilis is suggested by:[37]
CSF white blood cell (WBC) count >10 cells/mm³ (10 × 10⁶ cells/L)
CSF protein >50 mg/dL (0.50 g/L)
A positive CSF VDRL test.
The CSF will also demonstrate a positive TPHA, TPPA, or FTA-ABS treponemal test.[20][37][60] A non-reactive CSF-TPHA test result usually excludes neurosyphilis. Neurological involvement is unlikely at CSF TPHA or TPPA titres <1:320. There are no data to suggest a benefit to performing repeat cerebrospinal fluid examination in immunocompetent patients, or in patients with HIV who are on antiretroviral treatment, if serological and clinical responses are observed after the treatment for neurosyphilis.[8]
How to perform a diagnostic lumbar puncture in adults. Includes a discussion of patient positioning, choice of needle, and measurement of opening and closing pressure.
A chest x-ray should be performed in people with syphilis of unknown duration, and in those who have had syphilis for more than 2 years, whether or not they have had cardiac symptoms. This may detect possible aortic aneurysm or aortic calcification. Any patient with suspected aortic regurgitation, heart failure, or aortic aneurysm will require both a chest x-ray and echocardiogram.
All patients with syphilis should be tested for HIV. In geographical areas in which the prevalence of HIV is high, patients who have syphilis should be re-tested for HIV after 3 months, even if the first HIV test result is negative, and be offered HIV pre-exposure prophylaxis (PrEP).[8] Hence, a low threshold for the testing and treatment of syphilis in patients with HIV is advisable.
Initial investigations for congenital syphilis
The CDC has published recommendations concerning serological tests required in the diagnosis of congenital syphilis.[8] Syphilis serology should be performed on all pregnant women at the first antenatal visit, or as early as possible in pregnancy, and results interpreted in the same way as for non-pregnant individuals.[8][36][67] Serology should be repeated again at 28 weeks’ gestation and at delivery for women at high risk of syphilis infection.[8] Women who had no antenatal care before delivery or were at high risk for syphilis infection during pregnancy should have their serological status determined before they or their babies are discharged from hospital. No mother or baby should be discharged from hospital without the maternal serological status being documented at least once during the pregnancy.[8] In the context of the rapidly increasing rates of congenital syphilis, the American College of Obstetricians and Gynecologists (ACOG) recommends that all pregnant individuals should be screened serologically for syphilis at the first prenatal care visit, followed by universal rescreening during the third trimester and at birth, rather than a risk-based approach to testing.[68] All infants who are born to mothers with positive serology require a non-treponemal test (VDRL or RPR), which should be performed on the infant's serum rather than on umbilical cord blood. The infant’s non-treponemal test should be the same type of non-treponemal test performed on the mother.[8]
Any woman who delivers a stillborn infant after 20 weeks’ gestation should be tested for syphilis.
All pregnant women who have syphilis and all infants and children at risk for congenital syphilis should be tested for HIV; UK and US guidelines recommend testing for HIV as part of routine antenatal care.[8][69][70]
Further investigations for congenital syphilis
Pregnant women with syphilis or suspected of having syphilis require a fetal ultrasound scan. The presence of fetal or placental syphilis (e.g., hepatomegaly, ascites, hydrops fetalis) indicates a greater risk of treatment failure for congenital syphilis.[71]
After birth, lumbar puncture with CSF analysis for WBC count, protein, and VDRL, full blood count including differential and platelet count, long-bone radiographs, and other tests as clinically indicated (e.g., chest x-ray, liver function tests, neuroimaging, ophthalmological examination, and auditory brainstem response) are recommended by the CDC in the following cases:[8]
Neonates (aged <1 month) with confirmed proven or highly probable disease plus:
An abnormal physical examination that is consistent with congenital syphilis (e.g., non-immune hydrops, conjugated or direct hyperbilirubinaemia or cholestatic jaundice or cholestasis, hepatosplenomegaly, rhinitis, skin rash, or pseudoparalysis of an extremity) or
A serum quantitative non-treponemal serological titre that is fourfold higher than the mother's titre (e.g., maternal titre = 1:2, neonatal titre ≥1:8 or maternal titre = 1:8, neonatal titre ≥1:32) or
A positive dark-field test or PCR of placenta, cord, lesions or body fluids, or a positive visualisation of stained treponemal spirochetes in the placenta or cord using immunohistochemistry.
Neonates (aged <1 month) who have possible disease, with a normal physical examination and a serum quantitative non-treponemal serological titre the same or less than fourfold the maternal titre plus:
The mother was not treated, was inadequately treated, or has no documentation of having received treatment or
The mother was treated with erythromycin or other non-penicillin regimen or
The mother received treatment <4 weeks before delivery.
Children aged ≥1 month with reactive serological tests for syphilis.
Use of this content is subject to our disclaimer
