Although no single symptom or finding is diagnostic, clinical suspicion for endometriosis is generally sufficient for presumptive diagnosis.[4]Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020 Mar 26;382(13):1244-56.
http://www.ncbi.nlm.nih.gov/pubmed/32212520?tool=bestpractice.com
Clinical evaluation
A history of painful menstrual cramps (dysmenorrhoea), especially if unrelieved by non-steroidal anti-inflammatories (NSAIDs), is fairly suggestive of the diagnosis.[13]Laufer MR, Goitein L, Bush M, et al. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J Pediatr Adolesc Gynecol. 1997 Nov;10(4):199-202.
http://www.ncbi.nlm.nih.gov/pubmed/9391902?tool=bestpractice.com
Primary dysmenorrhoea (menstrual cramps that occur in the absence of obvious pelvic pathology) is extremely common in younger women.[1]American College of Obstetricians and Gynecologists. Dysmenorrhea and endometriosis in the adolescent. ACOG committee opinion no. 760. Obstet Gynecol. 2018 Dec;132(6):e249-58 (reaffirmed 2021).
https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/12/dysmenorrhea-and-endometriosis-in-the-adolescent
http://www.ncbi.nlm.nih.gov/pubmed/30461694?tool=bestpractice.com
Pain characterised as progressively worsening and continuous, however, is most characteristic for women with endometriosis.
Women may present with a spectrum of symptoms, including various genitourinary (e.g., dysuria, flank pain, haematuria) and gastrointestinal (e.g., dyschezia, haematochezia) complaints.[17]European Society of Human Reproduction and Embryology Endometriosis Guideline Development Group. Guideline on the management of women with endometriosis. Feb 2022 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline.aspx
[31]Society of Obstetricians and Gynaecologists of Canada. Clinical practice guideline - endometriosis: diagnosis and management. July 2010 [internet publication].
https://www.jogc.com/article/S1701-2163(16)34589-3/abstract
Commonly, women may also describe deep dyspareunia or pain on deep penetration during intercourse.[5]Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Invest. 2017;82(5):453-61.
http://www.ncbi.nlm.nih.gov/pubmed/27820938?tool=bestpractice.com
The diagnosis should also be considered in women presenting with unexplained sub-fertility.[33]Tanahatoe SJ, Hompes PG, Lambalk CB. Investigation of the infertile couple: should diagnostic laparoscopy be performed in the infertility work up programme in patients undergoing intrauterine insemination? Hum Reprod. 2003 Jan;18(1):8-11.
http://humrep.oxfordjournals.org/cgi/content/full/18/1/8
http://www.ncbi.nlm.nih.gov/pubmed/12525433?tool=bestpractice.com
Endometriosis is present in up to 40% of women presenting with unexplained sub-fertility.[33]Tanahatoe SJ, Hompes PG, Lambalk CB. Investigation of the infertile couple: should diagnostic laparoscopy be performed in the infertility work up programme in patients undergoing intrauterine insemination? Hum Reprod. 2003 Jan;18(1):8-11.
http://humrep.oxfordjournals.org/cgi/content/full/18/1/8
http://www.ncbi.nlm.nih.gov/pubmed/12525433?tool=bestpractice.com
These women may otherwise be asymptomatic.
Several demographic and anthropometric measures such as white ethnicity, low body mass index, and social behaviours (late first sexual encounters, smoking) have been weakly associated with endometriosis.[11]Missmer SA, Hankinson SE, Spiegelman D, et al. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004 Nov;104(5 Pt 1):965-74.
http://www.ncbi.nlm.nih.gov/pubmed/15516386?tool=bestpractice.com
[18]Bougie O, Yap MI, Sikora L, et al. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG. 2019 Aug;126(9):1104-15.
http://www.ncbi.nlm.nih.gov/pubmed/30908874?tool=bestpractice.com
Depression and anxiety appear to be associated with endometriosis, especially if chronic pain is present.[34]Lorencatto C, Petta CA, Navarro MJ, et al. Depression in women with endometriosis with and without chronic pelvic pain. Acta Obstet Gynecol Scand. 2006;85(1):88-92.
http://www.ncbi.nlm.nih.gov/pubmed/16521687?tool=bestpractice.com
[35]van Barneveld E, Manders J, van Osch FHM, et al. Depression, anxiety, and correlating factors in endometriosis: a systematic review and meta-analysis. J Womens Health (Larchmt). 2022 Feb;31(2):219-30.
http://www.ncbi.nlm.nih.gov/pubmed/34077695?tool=bestpractice.com
Therefore, women presenting with endometriosis should be assessed for comorbid mood or anxiety disorders. A multi-system approach that considers physical and psychological perspectives is required. See Depression in adults and Generalised anxiety disorder.
Enquire about a possible family history of endometriosis, as well as whether the woman misses work or school because of debilitating pain. Having a first-degree relative with a history of endometriosis increases the likelihood of endometriosis.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Absenteeism from work or school, along with a positive family history of endometriosis, is strongly correlated with a diagnosis of endometriosis.[37]Verket NJ, Falk RS, Qvigstad E, et al. Development of a prediction model to aid primary care physicians in early identification of women at high risk of developing endometriosis: cross-sectional study. BMJ Open. 2019 Dec 4;9(12):e030346.
https://bmjopen.bmj.com/content/9/12/e030346.long
http://www.ncbi.nlm.nih.gov/pubmed/31806607?tool=bestpractice.com
A gentle and thorough examination may help distinguish endometriosis from other pelvic pain disorders. Single digit pelvic examination, followed by bi-manual and rectovaginal examinations may reveal pelvic mass (ovarian endometrioma), fixed and retroverted uterus or uterosacral ligament nodularity and tenderness.[17]European Society of Human Reproduction and Embryology Endometriosis Guideline Development Group. Guideline on the management of women with endometriosis. Feb 2022 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline.aspx
Inspection and palpation of the abdomen is also recommended.[17]European Society of Human Reproduction and Embryology Endometriosis Guideline Development Group. Guideline on the management of women with endometriosis. Feb 2022 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline.aspx
[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Clinical examination may be normal in women with endometriosis.[17]European Society of Human Reproduction and Embryology Endometriosis Guideline Development Group. Guideline on the management of women with endometriosis. Feb 2022 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline.aspx
[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Physical examination should be decided on a case-by-case basis, and with informed patient consent. The National Institute for Health and Care Excellence (NICE) in the UK recommends that an abdominal examination should still be offered (to exclude abdominal masses) if a pelvic examination is declined or is unsuitable.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Ancillary studies
Transvaginal ultrasound is the imaging modality of choice to assess for the presence of endometriosis.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
[38]The American College of Obsteticians and Gynecologists. Practice bulletin no. 114: management of endometriosis. Obstet Gynecol. 2010 Jul;116(1):223-36.
http://www.ncbi.nlm.nih.gov/pubmed/20567196?tool=bestpractice.com
Transvaginal ultrasound may not detect early disease. Sensitivity and specificity for detecting endometriomas is 93% and 96%, respectively.[39]Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016 Feb 26;(2):CD009591.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD009591.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/26919512?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Ultrasound of ovarian endometriomaFrom the collection of Dr Jonathon Solnik; used with permission [Citation ends].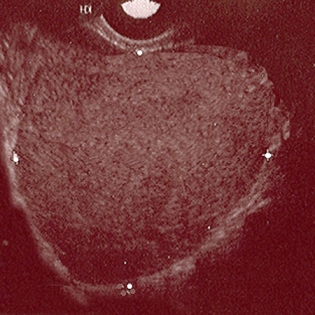
Transvaginal ultrasound has high specificity, but limited sensitivity, for diagnosis of vaginal, bladder, parametrium, rectovaginal septum, and uterosacral ligament endometriosis.[40]Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015 Nov;46(5):534-45.
https://obgyn.onlinelibrary.wiley.com/doi/epdf/10.1002/uog.15667
http://www.ncbi.nlm.nih.gov/pubmed/26250349?tool=bestpractice.com
[41]Gerges B, Li W, Leonardi M, et al. Meta-analysis and systematic review to determine the optimal imaging modality for the detection of bladder deep endometriosis. Eur J Obstet Gynecol Reprod Biol. 2021 Jun;261:124-33.
https://www.doi.org/10.1016/j.ejogrb.2021.04.030
http://www.ncbi.nlm.nih.gov/pubmed/33932683?tool=bestpractice.com
[42]Guerriero S, Martinez L, Gomez I, et al. Diagnostic accuracy of transvaginal sonography for detecting parametrial involvement in women with deep endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021 Nov;58(5):669-76.
https://www.doi.org/10.1002/uog.23754
http://www.ncbi.nlm.nih.gov/pubmed/34358386?tool=bestpractice.com
[43]Zhou Y, Su Y, Liu H, et al. Accuracy of transvaginal ultrasound for diagnosis of deep infiltrating endometriosis in the uterosacral ligaments: Systematic review and meta-analysis. J Gynecol Obstet Hum Reprod. 2021 Mar;50(3):101953.
https://www.doi.org/10.1016/j.jogoh.2020.101953
http://www.ncbi.nlm.nih.gov/pubmed/33148442?tool=bestpractice.com
For the diagnosis of rectosigmoid deep endometriosis, the specificity is 97% and the sensitivity is 89%.[44]Gerges B, Li W, Leonardi M, et al. Optimal imaging modality for detection of rectosigmoid deep endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021 Aug;58(2):190-200.
https://www.doi.org/10.1002/uog.23148
http://www.ncbi.nlm.nih.gov/pubmed/33038269?tool=bestpractice.com
Rectal endoscopic ultrasound may be considered in women with suspected deep pelvic endometriosis or involvement of the colon/rectum, as this may help plan surgical resection.[39]Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016 Feb 26;(2):CD009591.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD009591.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/26919512?tool=bestpractice.com
The 'sliding sign' can be used to assess for posterior cul-de-sac obliteration. A negative sliding sign is documented when the rectosigmoid colon does not slide smoothly over the posterior uterus/cervix. A negative sliding sign is both sensitive and specific for deep endometriosis and posterior cul-de-sac obliteration.[45]Reid S, Espada M, Lu C, et al. To determine the optimal ultrasonographic screening method for rectal/rectosigmoid deep endometriosis: ultrasound "sliding sign," transvaginal ultrasound direct visualization or both? Acta Obstet Gynecol Scand. 2018 Nov;97(11):1287-92.
https://obgyn.onlinelibrary.wiley.com/doi/10.1111/aogs.13425
http://www.ncbi.nlm.nih.gov/pubmed/30007066?tool=bestpractice.com
In the UK, NICE recommends that additional investigations and referral (if necessary) should be carried out concurrently, and alongside starting initial pharmacological management.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
This is to reduce delays in diagnosis.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
They recommend that all patients with suspected endometriosis should be offered a transvaginal ultrasound (organised by the patient’s general practice), even if the physical examination is normal.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
If a transvaginal ultrasound is unsuitable or declined by the patient, NICE recommends that a transabdominal ultrasound of the pelvis should be considered.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
They noted that although there was much less evidence on the transabdominal approach versus transvaginal, it was still necessary to provide an alternative option for when the latter is declined or unsuitable.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
The transvaginal ultrasound is used to:[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Identify ovarian endometriomas and deep endometriosis (including that involving the bowel, bladder or ureter)
Identify or rule out alternative pathologies
Guide management and enable referral to an appropriate service. Note that a normal ultrasound scan does not exclude endometriosis and referral may still be appropriate.
Magnetic resonance imaging (MRI) may be considered in selected patients.[17]European Society of Human Reproduction and Embryology Endometriosis Guideline Development Group. Guideline on the management of women with endometriosis. Feb 2022 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline.aspx
[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
MRI can detect extra-pelvic and rectovaginal implants. MRI (or specialist transvaginal ultrasound) may be used to diagnose and assess the extent of deep endometriosis.[17]European Society of Human Reproduction and Embryology Endometriosis Guideline Development Group. Guideline on the management of women with endometriosis. Feb 2022 [internet publication].
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline.aspx
[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
NICE advises that these scans should be planned and interpreted by a professional with specialist expertise in gynaecological imaging.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
[Figure caption and citation for the preceding image starts]: MRI - fibrotic nodules involving the uterosacral ligaments and rectal wallBazot M, et al. Radiology. 2004 Aug;232(2):379-89; used with permission [Citation ends].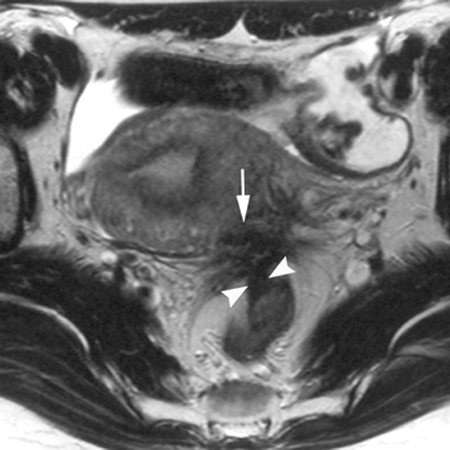
MRI, 3D ultrasonography or hysterosalpingography are ideal for imaging women with mullerian anomalies (e.g., transverse vaginal septum) or for identifying any scarring or tubal blockage causing outflow tract obstruction (e.g., in those with sub-fertility).
MRI or specialist pelvic ultrasound may also be used prior to operative laparoscopy in patients with suspected deep endometriosis.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Normal imaging (e.g., ultrasound, MRI) does not exclude endometriosis.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Serological markers such as CA-125 lack specificity and have not been shown to be useful diagnostic tools, while studies for other biomarkers are ongoing.[46]Nisenblat V, Bossuyt PM, Shaikh R, et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016 May 1;(5):CD012179.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD012179/full
http://www.ncbi.nlm.nih.gov/pubmed/27132058?tool=bestpractice.com
[47]Gupta D, Hull ML, Fraser I, et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016 Apr 20;(4):CD012165.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD012165/full
http://www.ncbi.nlm.nih.gov/pubmed/27094925?tool=bestpractice.com
NICE recommends referring patients to gynaecology for further investigation and management if they have any of the following:[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
symptoms of endometriosis which have a detrimental impact on their daily functioning or which are persistent or recurrent
pelvic signs of endometriosis (without suspected deep endometriosis)
suspected or confirmed endometrioma, deep endometriosis, or endometriosis outside the pelvic cavity. These patients should be referred to a specialist endometriosis service.
NICE recommends referring patients aged under 18 with suspected or confirmed endometriosis to a paediatric and adolescent gynaecology service or specialist endometriosis service.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Surgical findings
Surgical inspection with histopathological confirmation remains the definitive test for diagnosis, although a negative histological result does not exclude endometriosis, and up to 50% of peritoneal biopsies obtained during laparoscopy for pelvic pain show no evidence of disease.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
[48]Marchino GL, Gennarelli G, Enria R, et al. Diagnosis of pelvic endometriosis with use of macroscopic versus histologic findings. Fertil Steril. 2005 Jul;84(1):12-5.
http://www.ncbi.nlm.nih.gov/pubmed/16009147?tool=bestpractice.com
The use of preoperative imaging is associated with decreased morbidity and mortality and can aid patient decision making, surgical planning, and management.[49]American College of Radiology. ACR appropriateness criteria: endometriosis. 2024 [internet publication].
https://acsearch.acr.org/docs/3195150/Narrative
Symptom severity may not correlate with the extent of disease seen on careful surgical inspection. Not all women require surgical investigation. However, some physicians feel that if first line medical treatments (oral contraceptive pills, NSAIDs) fail, or if signs and symptoms are highly suspicious for endometriosis at initial evaluation, proceeding to surgery is an appropriate early measure. Diagnostic laparoscopy may be considered for suspected endometriosis even if other investigations (e.g., imaging) have been normal.[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
Laparoscopic evaluation is the preferred approach given the shorter recovery time compared with exploratory laparotomy. Surgical treatment can be simultaneously performed (with prior patient consent).[36]National Institute for Health and Care Excellence (UK). Endometriosis: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng73
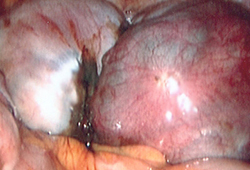
Operative findings widely vary and women should be staged according to the extent and type of lesions; size and depth of peritoneal/ovarian implants; and presence and extent of pelvic adhesions and degree of cul-de-sac obliteration.[50]Schenken RS, Guzick DS. Revised endometriosis classification: 1996. Fertil Steril. 1997 May;67(5):815-6.
http://www.ncbi.nlm.nih.gov/pubmed/9130883?tool=bestpractice.com
Early stage (minimal to mild) is marked by superficial peritoneal implants that appear vesicle-like (clear or red). These can be isolated or scattered and are more common in adolescents.
Moderate disease is typically characterised by multiple superficial or deep lesions with a variable degree of adhesions.
Advanced disease is typified by multiple implants (deep and fibrotic), parametrial or retroperitoneal extension, ovarian endometrioma, an obliterated cul-de-sac, and pelvic adhesions.[Figure caption and citation for the preceding image starts]: Laparoscopic image of ovarian endometriomaFrom the collection of Dr Jonathon Solnik; used with permission [Citation ends].