Recommendations
Urgent
Think 'Could this be sepsis?'
See our topics Sepsis in adults and Sepsis in children. Refer to your local sepsis protocols.
For any patient with suspected osteomyelitis:
Aim to take blood cultures before commencing antibiotics.[2][6]
Start intravenous broad-spectrum antibiotic therapy within 1 hour if high risk for sepsis.
In the patient with suspected native vertebral osteomyelitis and deteriorating neurological signs, seek urgent surgical review.
Do not give empirical antimicrobial therapy until a microbiological diagnosis is established if the patient has a normal and stable neurological examination, and stable haemodynamics.[7]
Consider which care setting would be most appropriate for your patient and refer to a multidisciplinary team according to your local protocol.
Admit any patient who is systemically unwell to hospital for intravenous antibiotic therapy.
If your patient is well, intravenous antibiotic therapy may be given through an ambulatory care pathway.
If your patient has diabetes and you have a clinical concern about bone infection, particularly in the patient's feet, inform the multidisciplinary foot care service immediately as this is a limb-threatening or life-threatening diabetic foot problem.[26]
Key Recommendations
In the patient with acute osteomyelitis:
If indicated, take blood cultures before starting antibiotics[2][6]
Follow local protocols for empirical antibiotic choice
Give a short course of intravenous therapy initially, and then switch to oral antibiotics when clinically indicated.
Once the diagnosis has been confirmed and results of cultures and sensitivities are known, modify the antibiotic regimen accordingly.
Generally, give antibiotics for 6 weeks in total.
Offer analgesia
Immobilise the limb for comfort if required
Monitor and manage comorbidities during the hospital stay
Seek specialist advice on the need for surgical management (e.g., debridement or drainage of abscesses), in addition to antibiotic therapy.
In the patient with suspected native vertebral osteomyelitis, seek advice from an infectious disease specialist and a spine surgeon.[7]
In practice, once you have informed the multidisciplinary foot care service about any patient with diabetes and a suspected foot bone infection, see your local treatment protocol and discuss the patient with microbiology in order to start an appropriate antibiotic regimen.
Refer all patients with chronic osteomyelitis for specialist management.
Comorbidities may affect management and prognosis.
Care is often complex, requiring referral to a specialised bone infection unit and multidisciplinary team input.
Include the patient in decisions on the best course of management, weighing up the risks versus the benefits of treatment. Options may include:
Complex curative surgery, antibiotic therapy, reconstructive surgery, and rehabilitation
Limited surgery to reduce the infective load rather than aiming for a cure
Suppression - withholding full curative management and treating flare-ups with short antibiotic courses only.
Practical tip
Think 'Could this be sepsis?
See our topics Sepsis in adults and Sepsis in children.
Refer to local guidelines for the recommended approach at your institution for assessment and management of the patient with suspected sepsis.
Refer to your local paediatric sepsis protocol.
When managing acute osteomyelitis, apply the following general principles:
Follow local microbiology guidelines
Aim to take blood cultures before starting intravenous antibiotic therapy[2][6]
In a patient with suspected native vertebral osteomyelitis who has a normal and stable neurological examination and stable haemodynamics, do not give empirical antimicrobial therapy until a microbiological diagnosis is established.[7]
In practice, if the patient is unwell, give antibiotics within 1 hour of them reaching hospital
If the patient is systemically well, wait for results of the culture before giving targeted intravenous antibiotic therapy
However, if the patient has a diabetic foot problem, you should not delay giving antibiotics (see section Suspected osteomyelitis in a patient with a diabetic foot problem, below, for details).
If there is a risk of atypical organisms, seek advice from your microbiology department (e.g., a patient with sickle cell disease requires cover for salmonella; penetrating or waterborne injuries may require cover for Pseudomonas)
Treat with a short course of intravenous antibiotic therapy, and then switch to oral therapy when clinically indicated.[6]
Take account of antibiotic susceptibilities demonstrated in organism cultures.[6]
Antibiotics are usually sufficient to treat acute bone infection (i.e., surgical procedures are usually unnecessary) if all of the following apply:
The diagnosis is made within a few days of symptom onset
No dead bone or abscesses are present on imaging
Rapid response is seen to systemic antibiotic treatment
There is no associated septic arthritis.
If any diagnosis is delayed, dead bone or abscesses are present, response to antibiotics is slow, or a septic arthritis is associated with the osteomyelitis, additional treatment, such as drainage or surgery, will often be required.
Practical tip
Note that vertebral osteomyelitis without cord compression and tuberculous osteomyelitis usually do not require surgical intervention.
Admission
Admit any patient who is systemically unwell to hospital for intravenous antibiotic therapy.
If an adult patient is well, you can arrange for intravenous antibiotic therapy to be given through an ambulatory care pathway, depending on local resources.
Admit any child with acute osteomyelitis to hospital for intravenous antibiotic therapy, unless there are exceptional circumstances.[6]
Admit any patient with diabetes for strict glucose control, intravenous antibiotic therapy, and surgery if indicated. Inform the multidisciplinary foot care service if you have a clinical concern about a bone infection in the foot - they should take responsibility for managing the patient.[26]
Discuss admission of a patient with an acute exacerbation of chronic osteomyelitis with the team that usually provides their care.
Antibiotic therapy
Establish the aetiology of infection and classify the disease to plan individual treatment. Follow local protocols.
Base initial antibiotic choice on the most likely causative organism, which in turn depends on:
The patient’s age
Immunisation history
Comorbidities
The prevalence of organisms in the community, and their antimicrobial sensitivities.
Once the organism is identified by culture or PCR, and sensitivity to antibiotics is determined, narrow antibiotic therapy accordingly.
Route of administration and duration of therapy
The optimal duration of antimicrobial therapy is not certain.[63] In practice, continue antibiotic treatment for about 6 weeks in total.
Evidence: Route of antibiotic administration
Oral antibiotics appear to be as effective as intravenous antibiotics for the treatment of osteomyelitis, usually following surgical debridement and a short initial course of intravenous antibiotics. The oral route also helps to avoid complications associated with longer-term intravenous access.
A Cochrane systematic review (search date October 2012) on antibiotics for chronic osteomyelitis in adults identified four small RCTs (n=150) comparing oral and intravenous routes of administration.[64]
The majority of people were male and had lower limb post-traumatic infection.
All trials mentioned surgical debridement prior to starting antibiotics, although in two trials it was unclear if all patients underwent debridement.
Duration of treatment varied between studies and between individual participants within studies. Follow-up varied from 6 months to 39 months.
All studies were at high risk of bias in at least one domain, three had significant loss to follow-up.
There was no difference in remission rates at the end of treatment (4 trials; 70/80 with oral vs. 58/70 with intravenous; RR 1.04 [95% CI 0.92 to 1.18]) or at 12 months after treatment (3 trials; 49/64 with oral vs. 44/54 with intravenous; RR 0.94 [95% CI 0.78 to 1.13]).
There was also no difference in adverse events.
The Oral Versus IntraVenous Antibiotics (OVIVA) trial (published 2019) was a UK multicentre, pragmatic RCT. It was open label; however, outcomes assessors were blinded.[63]
The trial included adults with acute or chronic bone or joint infections, specifically native osteomyelitis of the extra-axial skeleton, native joint infection requiring excision arthroplasty, prosthetic joint infection, orthopaedic fixation-device infection, or vertebral osteomyelitis with or without associated discitis or soft-tissue infection.
Patients had multiple bacterial pathogens and therefore many different antibiotics were used.
Randomisation to oral (n=466) or intravenous (n=443) groups was within 7 days of surgery or 7 days of starting antibiotics if no surgery.
Duration of treatment was 6 weeks after randomisation, although extended treatment was allowed if required clinically.
Choice of antibiotic was at the discretion of the treating clinician.
Follow-up was for 1 year.
For the primary outcome of treatment failure, there was no difference between oral or intravenous antibiotics (13.2% [oral] vs. 14.6% [intravenous]; risk difference -1.4% [95% CI -5.6% to +2.9%]).
This was also true for all subgroups including people with osteomyelitis (axial or extra-axial, with debridement OR 0.93 [95% CI 0.45 to 1.94]; without debridement OR 0.34 [95% CI 0.08 to 1.41]).
People in the intravenous group were more likely to have a longer length of hospitalisation and to discontinue their treatment early compared with the oral group.
Patients in the intravenous group experienced a significant increase in complications due to the intravenous catheter; however, serious adverse events and C difficile diarrhoea were similar between the oral and intravenous groups.
76.7% received extended therapy (beyond 6 weeks; 395 in intravenous group vs. 410 in oral; median duration of therapy 78 days [interquartile range 42 to 99 days] in the intravenous group and 71 days [interquartile range 43 to 94 days] in the oral group; P=0.63).
A multicentre, retrospective cohort study published in 2015 of 2060 children and adolescents with acute osteomyelitis compared oral antibiotics (n=1005) with intravenous antibiotics (administered by peripherally inserted central catheter [PICC]; n=1055) after hospital discharge (median length of stay 6 days).[65]
There was no difference in treatment failure between groups (risk difference 0.3% [95% CI −0.1% to +2.5%]).
In the PICC group there was a 15% complication rate requiring an emergency department visit, and therefore an increased risk of hospitalisation in this group.
Duration of therapy was similar in both groups (mean 32 days [interquartile range 28 to 37 days] with oral vs. 27 days [interquartile range 20 to 35 days] with intravenous treatment).
Supportive care
Offer pain relief to all patients as required. Start with paracetamol or a non-steroidal anti-inflammatory drug (NSAID) such as ibuprofen, with an opioid (e.g., morphine) for breakthrough pain relief. If regular use of morphine is required, switch to a slow-release oral formulation. In practice, severe pain usually suggests there is a collection (abscess) that needs to be drained.
Address any comorbidities. It is particularly important to maintain strict blood glucose control in any patient with diabetes and a major infection such as osteomyelitis.
Elevate and immobilise the affected limb if necessary.
Look out for deep vein thrombosis particularly with Staphylococcus aureus osteomyelitis.[6][66] Refer to a haematologist and follow local protocols. See our topic Deep vein thrombosis.
Consider referral to other specialist teams, such as:
The haematology team for a patient with sickle cell disease
The diabetes inpatient specialist team for a patient with diabetes mellitus but who does not have a diabetic foot problem.
For any patient with a limb-threatening or life-threatening diabetic foot problem, including a clinical concern that there is a bone infection, liaise with the multidisciplinary foot care service immediately.[26] See the section Suspected osteomyelitis in a patient with a diabetic foot problem, below, and our topic Diabetic foot complications, for more information.
Surgery
A rapid response to antibiotic treatment is usually expected, but if the limb deteriorates or imaging suggests progressive bone destruction, discuss choice of antibiotics with microbiology and consider surgery to prevent progression to chronic osteomyelitis.
Although acute osteomyelitis often responds to antibiotics alone if it can be treated promptly and aggressively, once dead bone or a biofilm has become established, antibiotics alone cannot cure the infection and thorough surgical debridement is required.
Ensure drainage of fluid collections, if present, by radiological guidance or surgery.
If you are considering surgery, seek advice from orthopaedics.
Choice of antibiotics depends on the likely cause and the prevalence of antimicrobial resistance.
Empirical antibiotic therapy for adults
Low MRSA prevalence (<10%)
In areas of low MRSA prevalence, give intravenous flucloxacillin. If the patient has a penicillin allergy, give intravenous vancomycin.
High MRSA prevalence (>10%-15%)
In areas of high MRSA prevalence, give intravenous vancomycin. Teicoplanin and linezolid are reasonable alternatives if the patient cannot tolerate vancomycin, or if vancomycin is contraindicated.
Pseudomonas cover
Walking barefoot may predispose children, particularly, but not exclusively, to osteomyelitis from penetrating injury. Pseudomonas can also be waterborne.
In UK practice, use piperacillin/tazobactam in the first instance if you suspect infection with Pseudomonas.
If the patient is allergic to penicillin, use a fluoroquinolone such as ciprofloxacin (consider safety issues - see below).[6] Meropenem is another alternative.
Empirical antibiotic therapy for children
Low MRSA prevalence (<10%)
For children aged 3 months to 5 years, give an intravenous cephalosporin such as cefazolin or cefuroxime.[6] In UK practice, ceftriaxone is considered a better option for methicillin-sensitive S aureus (MSSA) than cefuroxime.
First-generation cephalosporins (e.g., cefazolin) may be suboptimal if the osteomyelitis is caused by Streptococcus pneumoniae or Haemophilus influenzae type b in unvaccinated children.[6]
While second-generation cephalosporins (e.g., cefuroxime) and third-generation cephalosporins (e.g., ceftriaxone) demonstrate better coverage for S pneumoniae and H influenzae, they may be inferior to first-generation cephalosporins for osteomyelitis caused by S aureus.[6] In the UK, it is generally accepted that ceftriaxone provides adequate cover against MSSA.
Alternative options include amoxicillin/clavulanate, ceftriaxone, an antistaphylococcal penicillin (e.g., flucloxacillin), or clindamycin (for non-Kingella species). An antistaphylococcal penicillin is not effective against Kingella kingae.[6]
For children aged ≥5 years, give an intravenous antistaphylococcal penicillin, or cefazolin.[6]
When risk factors for atypical organisms are present (e.g., sickle cell disease) consider ceftriaxone with or without an antistaphylococcal penicillin or clindamycin.[6]
High MRSA prevalence (>10%-15%)
For children aged <2 years, add clindamycin to the empirical regimen as indicated for low MRSA prevalence above.[6] If, however, the prevalence of clindamycin resistance in MRSA is >10%, prescribe vancomycin or linezolid.[6]
For children aged ≥2 years, give clindamycin alone, as long as the prevalence of clindamycin resistance in MRSA is <10%.[6]
Rifampicin or an antistaphylococcal beta-lactam (e.g., amoxicillin/clavulanate) may be added depending on patient and prevalence factors.[6]
If there is severe infection, a high-rate of clindamycin resistance (≥10%), or initial treatment failure, treat with vancomycin or teicoplanin. Rifampicin may be added; however, there is little evidence to support this.[6]
Always consider adding a beta-lactam until MRSA is confirmed to be the causative organism.[6]
Pseudomonas cover
Walking barefoot may predispose children, particularly, but not exclusively, to osteomyelitis from penetrating injury. Pseudomonas can also be waterborne.
In UK practice, use piperacillin/tazobactam in the first instance if you suspect infection with Pseudomonas.
If the patient is allergic to penicillin, use a fluoroquinolone such as ciprofloxacin (consider safety issues - see below).[6] Meropenem is another alternative.
In practice, take into consideration the age of the child, local organism prevalence, their sensitivities to antibiotic regimens, and consult local protocols.
Consider switching from intravenous to oral antibiotics after 2 to 4 days when the child:[6]
Is afebrile for 24 to 48 hours
Shows clinical improvement with reduced pain, inflammation, and improved mobility
Has a C-reactive protein (CRP) level that has decreased by at least 30% of the highest recorded value.
Drug safety alert: Restrictions on the use of fluoroquinolone antibiotics
In November 2018, the European Medicines Agency (EMA) completed a review of serious, disabling, and potentially irreversible adverse effects associated with systemic and inhaled fluoroquinolone antibiotics. These adverse effects include tendonitis, tendon rupture, arthralgia, neuropathies, and other musculoskeletal or nervous system effects.
As a consequence of this review, the EMA now recommends that fluoroquinolone antibiotics be restricted for use in serious, life-threatening bacterial infections only. Furthermore, it recommends that fluoroquinolones should not be used for mild to moderate infections unless other appropriate antibiotics for the specific infection cannot be used, and should not be used in non-severe, non-bacterial, or self-limiting infections. Patients who are older, have renal impairment, or have had a solid organ transplant, and those being treated with a corticosteroid are at a higher risk of tendon damage. Coadministration of a fluoroquinolone and a corticosteroid should be avoided.[67] The UK-based Medicines and Healthcare products Regulatory Agency (MHRA) supports these recommendations.[68]
Pathogen-targeted antibiotic therapy
Once the organism is identified by culture or PCR, and sensitivity to antibiotics is determined, narrow antibiotics accordingly.
The European Society For Paediatric Infectious Diseases (ESPID) guideline on bone and joint infections provides detailed information regarding targeted antibiotic therapy in children.[6]
Practical tip
Note that necrotising features, multifocal osteomyelitis, and recurrence may be caused by Panton-Valentine leukocidin (PVL)-producing S aureus and is associated with serious risk. The British Society for Antimicrobial Chemotherapy (BSAC) makes antibiotic recommendations for this rare aetiology.[56][69]
Treat children usually for an average total duration of 3 to 4 weeks. In children who respond well, early transition from intravenous to oral therapy (after 3 days to 1 week) may be as effective as longer courses of intravenous treatment.[70][71] Signs suggestive of clinical improvement include:[6]
Apyrexia
Increased mobility
Decreased inflammation
Decreased pain
Decreasing CRP.
Treat complex osteomyelitis in children with longer courses of both intravenous and oral antibiotics. Suspect complex osteomyelitis in the following situations:[6]
Significant bone destruction or complications, such as abscesses
Salmonella, MRSA, or Panton-Valentine leukocidin (PVL)-positive strains
Young infants
Slow clinical improvement
Pelvic or spinal involvement.
Surgery
Ensure drainage of fluid collections, if present, by radiological guidance or surgery.
For adults who may require surgery, consult a specialist orthopaedic surgeon.
In children, consider surgery in the following situations:[6]
Persistent or recurring fever after 3 to 4 days
Periosteal abscess with persistent fever and raised CRP
Sequestration
MRSA or Panton-Valentine leukocidin (PVL)-positive S aureus
Chronic osteomyelitis
Prosthetic material.
Seek paediatric orthopaedic advice.
For supportive care and general surgical principles, see the section Suspected acute osteomyelitis: general principles above.
Empirical antibiotic therapy for adults
For the patient with suspected native vertebral osteomyelitis, and who is haemodynamically unstable, has progressive or severe neurological signs, or has signs of sepsis, give an antibiotic regimen that covers staphylococci (including MRSA), streptococci, and gram-negative bacilli.[7]
Vancomycin plus a third- or fourth-generation cephalosporin (e.g., ceftriaxone) is an example of a suitable regimen.[7]
For patients with an allergy or intolerance, give daptomycin plus a fluoroquinolone such as ciprofloxacin (consider safety issues - see Drug safety alert panel, above).[7]
Note that these regimens are based on Infectious Diseases Society of America (IDSA) guidance.[7] UK regimens may be narrower spectrum due to less resistance; consult local protocols because no national guidance for native vertebral osteomyelitis has been published.
Do not prescribe empirical antifungal and antimycobacterial therapy in most situations.[7]
Practical tip
If you suspect native vertebral osteomyelitis, consider seeking advice from an infectious disease specialist and a spine surgeon.[7] If neurological signs deteriorate, seek urgent surgical review.
Empirical antibiotic therapy for children
For children with spondylodiscitis or vertebral osteomyelitis, seek advice from microbiology on empirical antibiotic regimens and doses, and from an infectious disease specialist.
Pathogen-targeted antibiotic therapy
Tailor antibiotic therapy to culture and sensitivity results.
Give a total duration of 6 weeks of parenteral or highly bioavailable oral antimicrobial therapy. Detailed guidance on regimens is available from the IDSA.[7]
When the aetiological agent is Brucella, culture may be difficult and results slow to obtain, as the organism is intracellular and the number of circulating bacteria is usually low.[72] Therefore, targeted antibiotic therapy may need to be started when PCR results are available and infection has been confirmed, regardless of whether culture and sensitivity results are available. Follow local antibiotic protocols or seek advice from microbiology on choice of antibiotic regimen in endemic areas. Give antibiotics for Brucella infection for 3 months.[7] See our Brucellosis topic for more information. Seek evaluation by a spine surgeon and an infectious disease specialist in non-endemic areas.[7]
Surgery
Surgical intervention is required for progressive neurological deficits, progressive deformity, and spinal instability with or without pain despite adequate antimicrobial therapy.[7]
Surgical debridement with or without stabilisation is needed for persistent or recurrent bloodstream infection (without alternative source) or worsening pain despite appropriate medical therapy.[7]
In practice, if you suspect spondylodiscitis or vertebral osteomyelitis in a child, seek advice from paediatrics and/or paediatric orthopaedics, depending on local resources.
For general surgical principles and supportive care, see Suspected acute osteomyelitis: general principles section above.
Refer the patient to the multidisciplinary foot care service within 24 hours of the initial examination of the patient’s feet.[26]
Start empirical antibiotic therapy in patients with suspected diabetic foot infection as soon as possible.[26]
Take samples for microbiological testing before, or as close as possible to, the start of antibiotic therapy.[26]
Practical tip
In a patient with a limb-threatening or life-threatening diabetic foot problem, including a clinical concern that there is a bone infection, immediately inform the multidisciplinary foot care service so that the patient can be assessed and an individualised treatment plan can be put in place.[26] In practice, see your local treatment protocol and discuss the patient with the multidisciplinary foot care service and/or microbiology in order to start an appropriate antibiotic regimen.
When choosing an empirical antibiotic regimen for suspected osteomyelitis in a patient with a diabetic foot problem, take into account:[26]
The risk of developing complications
Previous microbiological results
Previous antibiotic use
Patient preferences.
Empirical antibiotic therapy for adults
For suspected osteomyelitis (moderate to severe diabetic foot problem) in an adult aged ≥18 years, prescribe one of the following antibiotic regimens intravenously for 48 hours until the patient is stabilised:[26]
Flucloxacillin with or without gentamicin and/or metronidazole
Amoxicillin/clavulanate with or without gentamicin
Ceftriaxone plus metronidazole.
In patients with a penicillin allergy, prescribe trimethoprim/sulfamethoxazole with or without gentamicin and/or metronidazole.[26]
If you suspect Pseudomonas infection, add in:[26]
Piperacillin/tazobactam
Clindamycin plus ciprofloxacin (consider safety issues - see Drug safety alert panel above) and/or gentamicin, if the patient has a penicillin allergy.
For MRSA, give one of the following:[26]
Vancomycin
Teicoplanin
Linezolid (if vancomycin or teicoplanin cannot be used; specialist use only).
Review intravenous antibiotics at 48 hours and consider switching to oral antibiotics if possible.[26]
Empirical antibiotic therapy for children
Seek specialist advice when prescribing empirical antibiotic therapy for a suspected diabetic foot infection in children and young people aged <18 years.[26]
Pathogen-targeted antibiotic therapy
When microbiological results are available, review and change the antibiotic accordingly, using a narrow-spectrum antibiotic, if appropriate.[26]
Review intravenous antibiotic therapy at 48 hours and consider switching to oral antibiotics if possible.[26]
Treat osteomyelitis for 6 weeks.[26]
Review the need for continued antibiotics regularly.[26]
Surgery
The NICE guideline on the prevention and management of diabetic foot problems does not address surgery for osteomyelitis in the foot of a patient with diabetes.[26] Seek advice from the multidisciplinary foot care service.
For supportive care and general surgical principles, see Suspected acute osteomyelitis: general principles section above.
If acute osteomyelitis can be treated promptly and aggressively with antibiotics (and drainage of collections), the disease process can be arrested before necrotic bone develops, and progression to chronic osteomyelitis may be prevented. Once necrotic bone is present, or a biofilm has become established around an implant, antibiotic therapy alone cannot cure the infection and surgery is required.
Repeated suboptimal antibiotic treatment without surgery increases microbial resistance and restricts the choice of effective antibiotics available following surgery.
Chronic osteomyelitis in children is rare. When it does occur, discuss the patient with a paediatric orthopaedic surgeon and infectious diseases consultant.
Many adult patients with chronic osteomyelitis have lived with their condition for years because they have been told in the past that there is no surgical solution for their condition. Definitive treatment should not be unduly delayed, particularly in infected fractures and non-unions, because further soft-tissue injury can occur through ongoing bone instability. Within specialist centres, eradication of infection with limb salvage and the treatment of even complex infection is often successful.
[Figure caption and citation for the preceding image starts]: A 62-year-old man suffered an open tibial fracture, which became infected after internal fixation. He continued with intermittent discharge of pus from the front of his tibia for 21 years. Imaging confirmed the presence of chronic osteomyelitis with a central area of dead bone (sequestrum)Courtesy of the Oxford Bone Infection Unit; used with permission [Citation ends].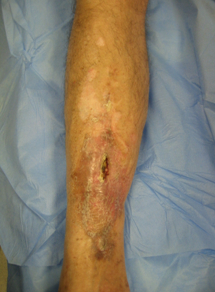
In practice, treat a patient with a flare-up in the same way as you would treat a patient with a purely acute presentation, but refer them to the team that usually supervises their care.
Discuss admission of a patient with an acute exacerbation of chronic osteomyelitis with the team that usually provides their care.
Suspected sepsis
Practical tip
Think 'Could this be sepsis?
See our topics Sepsis in adults and Sepsis in children.
Refer to local guidelines for the recommended approach at your institution for assessment and management of the patient with suspected sepsis.
Refer to your local paediatric sepsis protocol.
Give empirical broad-spectrum antibiotic therapy immediately, before debridement surgery.
If possible, obtain blood cultures or local pus samples for culture before starting antibiotic therapy.
Modify the regimen when the results of cultures and sensitivity tests are known.
Site of care
Consider where the patient should receive their treatment and discuss this with them. The optimal site of care will be influenced by patient factors and disease stage. See Classification (in the Aetiology section of this topic).
Owing to the many and varied comorbidities present in class B patients under the Cierny-Mader classification and the particular surgical techniques required to tackle complex infections, these patients should be seen in a specialist bone infection centre.
In patients with type IV disease, in diffuse osteomyelitis, or when there are infected fractures, a multidisciplinary team specialising in bone infection should be involved.
The team should include orthopaedic surgeons, microbiologists, plastic surgeons, and clinical nurse specialists who assist with home intravenous therapy and wound care.
Involve a musculoskeletal radiologist who can interpret imaging and perform percutaneous bone biopsies.
There is also a role for a vascular surgeon in a patient with peripheral vascular disease. For example, a patient with a non-union or a foot ulcer may require revascularisation prior to operative intervention to improve outcome.
Individual treatment
The outcome of surgery depends on the physiological status of the patient, the infection duration, microbiology, and extent of bone necrosis. It is essential to discuss options with the patient. See Classification (in the Aetiology section of this topic).
The decision on the best course of management for a patient with chronic osteomyelitis involves an assessment of:
The effects of the disease
The benefits of treatment to the patient
Associated risks.
As full cure of chronic osteomyelitis may involve complex surgery (with potential complications, antimicrobial drug reactions, staged reconstruction, and prolonged time in treatment and rehabilitation), an approach that controls current symptoms, but with the possibility of later recurrence, may be an option for some patients.
For a patient with disease classified as class C in the Cierny-Mader classification, it may be reasonable to withhold treatment or just to treat symptomatic flare-ups with short antibiotic courses. Select an appropriate antibiotic regimen based on results of radiologically guided biopsy. If the flare-ups are back to back with a matter of days between them, escalate the therapy to long-term antibiotic suppression.
In some cases, rather than achieving cure, limited surgery to reduce the infective load followed by long-term antibiotic suppression may be an option (e.g., when existing implants provide a challenging reconstructive problem).
Whatever the circumstances of the individual patient, functional rehabilitation is important.
Surgery
The approach to surgery can be guided by imaging, with plain film x-ray and magnetic resonance imaging (MRI) being particularly useful in accessing the sequestrum and avoiding unnecessary damage to the healthy bone when making a window to enter the medullary canal. MRI is also helpful in identifying localised active disease when the normal morphology of the bone is disrupted. A tourniquet is used wherever possible. Any sinus is excised with an ellipse of skin in the line of the incision.
Preoperative factors
These include:
Accurate clinical staging using the Cierny-Mader classification. See Classification (in the Aetiology section of this topic)
Diagnostic tests to assess general health and the condition of the limb (blood tests, scanning, angiography, guided biopsy)
Optimisation of associated medical comorbidities before surgery, including:
Nutritional status
Smoking
Substance dependence
Anaemia
Coagulopathies
Glucose control
Vascular insufficiency
Sickle cell disease needing exchange transfusion.
Operative principles
Surgery in a patient with chronic osteomyelitis is complex and often warrants technical expertise and multidisciplinary input. For specific details, see the sections below. In general, operative principles include:
Thorough debridement and excision of all infected tissue
Meticulous microbiological and histological sampling early during the procedure
Obtaining uncontaminated representative samples to diagnose the causative organism and rule out other potential differentials, such as tumour, is essential.
Dead space management to prevent the formation of haematoma
Haematoma increases infection recurrence rates.
Stabilisation of the bone, when there is instability or risk of fracture, usually with an external fixator
Attaining immediate soft-tissue coverage with healthy vascularised tissue that can deliver systemic antibiotics.
Postoperative priorities
These include:
Functional rehabilitation
Targeted antibiotic therapy
Close monitoring for early recurrence or adverse events
Second-stage reconstruction.
Microbiological sampling
To increase the accuracy of microbiological diagnosis, multiple samples should be taken, with 3 to 5 recommended as being a good balance between achieving sufficient sensitivity and avoiding poor specificity due to contamination.[2][73]
A clean set of instruments should be used for each sample and there should be no handling of the instrument tips by the surgeon or scrub staff. Every effort should be made to avoid contact with the skin of the patient, and contamination can be further reduced by using clean gauze swabs in the wound rather than using fingers or suction tips before sampling is complete.
Ideally these samples should be taken early in the operation when contamination of the wound is at its lowest.
Once taken, these samples should be sent directly to the laboratory to prevent undue sample degradation.
Samples for histology are also useful in supporting infection diagnosis as well as ruling out other potential conditions. Any long-standing sinus tracts should also be sent for histology to rule out the possibility of squamous cell malignant transformation.
Once sampling has been completed, antibiotic therapy is given. Initial intravenous antibiotic therapy appropriate to the flora encountered in the hospital’s region must be continued until a definitive regimen is selected based on the intraoperative culture results.
A protocol using a glycopeptide and a carbapenem antibiotic postoperatively in 166 cases of osteomyelitis debridement showed coverage of 96% of all organisms subsequently cultured.[74] This study revealed that one third of organisms cultured were resistant to a penicillin-based empirical antibiotic regimens. The carbapenem is usually stopped after 48 hours' culture if no gram-negative organisms have been cultured at that point.
If the patient does not respond to standard therapy, atypical organisms or fungal infections should be considered.
Surgical debridement
Sequestered bone is often found within the medullary canal because over time it is surrounded and encased by new reactive involucrum. See Classification (in the Aetiology section of this topic) for types of disease progression.
Type I Cierny-Mader disease
Medullary infection may be debrided through a cortical window placed in the metaphysis to limit the risk of subsequent fracture.
The edges of the window should be kept round to reduce the risk of developing a stress fracture.
There is often a layer of endosteal sequestrum on the inner surface of the cortex that must be removed back to bleeding bone.
If the medullary involvement is purely at the isthmus, it may be possible to use intramedullary reamers from one end of the bone without windowing the bone.
Type II Cierny-Mader disease
In Cierny-Mader type II disease, when the cortex is involved, the overlying soft tissues may need to be removed back to healthy cortical bone surface.
The abnormal cortex is brittle and discoloured, as opposed to normal bone, which has vascular periosteum that is adherent to the bone surface.
A chisel is used and the cortical layer is removed until bleeding bone is encountered.
Type III Cierny-Mader disease
In Cierny-Mader type III disease, a segment of healthy bone remains. However, careful preoperative planning is required to minimise the risk of fracture. It may be necessary to use a simple monolateral external fixator to support the bone following surgery until it is sufficiently healed. The medulla is reached by windowing the affected area of the bone and the endosteum is cleared along with any sequestra in the cortex, subperiosteal abscesses, involved soft tissues, and scarred skin.
At this stage it is important to focus on the adequacy of the debridement without concern about the later reconstructive challenges. This concern may prevent adequate excision and failure of infection control, rendering reconstruction ineffective.
Type IV Cierny-Mader disease
Type IV disease, by definition, requires a segmental resection to eliminate infection. The resection should also remove the involved soft tissues and skin but there should be care to ensure the neurovascular bundles are preserved.
There is a good case to apply an external fixator before the resection is undertaken to ensure the limb alignment and that stability is maintained.
This surgery is often best achieved as a combined approach between orthopaedic and plastic surgeons.
Dead space management
After debridement, the surgeon must assume that the whole operative field will be contaminated with bacteria disseminated during surgery.[75]
Bone is a rigid tissue so any defect left at the end of the surgery will remain and will fill with haematoma, providing an ideal environment for the propagation of persisting planktonic bacteria and allowing early biofilm development.
Systemic antibiotic therapy is administered in an effort to target these remaining organisms, but the perfusion of antibiotics into bone defects may be poor, resulting in low antibiotic penetration.
The dose of systemic antibiotics is also limited by potential toxicity on organs, such as the kidneys.
Dead space management options
Technique | Types | Description |
|---|---|---|
Soft tissue | Local soft tissue, such as a muscle flap (e.g., gastrocnemius) | Can obliterate shallow defects and facilitate the local delivery of high levels of parenteral antibiotics.[76] Covers small defects. Using muscle to fill deeper defects is often unsatisfactory and may prevent bone healing. |
Vascularised tissue free flap (e.g., ALT free flap) | Can obliterate deep defects and facilitate the local delivery of high levels of parenteral antibiotics.[76] Can cover large defects and increase union rates. | |
Antibiotic-containing materials | Can reduce the dead space and deliver high concentrations of local antibiotics (often 10 to 100 times the minimum inhibitory concentration of the organisms present.[77][78] This potentially provides antibiotics above the mean biofilm eradication concentration without systemic toxicity. | |
Antibiotic-containing polymethyl-methacrylate cement (PMMA) | PMMA can be used in the form of beads on a wire or moulded to fit the cavity.[79][80][81] The choice of antibiotics can be tailored to the cultured organisms, but it is important that the antibiotic is stable when mixed with cement. The beads or mould must be removed, because if left in place the PMMA will prevent bone ingrowth over time.[82] Secondary infection into the foreign material is a risk after the antibiotic has fully eluted.[83][84] | |
Biodegradable antibiotic carriers | These elute antibiotics at high concentrations but dissolve over several weeks. A range of biodegradable carriers is available. Some contain calcium sulfate and others contain compounds that dissolve at different rates. This negates the need for further surgery for removal once beads or moulds have served their purpose, and makes single-stage surgery for osteomyelitis a viable option. Outcomes for treating chronic osteomyelitis with ceramic antibiotic carriers in humans have been published.[85][86][87][88][89][90][91][92] Many are osteoconductive and this might aid bone healing. Wound ooze may occur, but is not predictive of infection recurrence and can be managed conservatively.[91][93] | |
Composite carriers | Composite carriers are available in a paste that sets to form a solid cold-curing cement. This gives the theoretical advantage of more initial structural support with the potential for osseous healing through osteoconduction as the carrier becomes more porous during dissolution.[94][95][96] | |
Acute shortening of the limb combined with a later limb lengthening | An effective way to manage type IV segmental defects. Acute shortening is safe in the tibia by up to 4 cm, the femur by 6 cm, and the humerus by 5 cm, assuming the soft tissues remain supple and no scars constrict the neurovascular bundles. The patient needs to be fit for reconstruction.[97] | |
Staged reconstruction in type IV osteomyelitis | The bone defect is filled with a temporary antibiotic-containing spacer and then the skin is closed. Once the infection has been treated and the soft tissues recovered, a second-stage procedure is performed to remove the spacer and reconstruct the defect. | |
Masquelet technique | A specific staged reconstruction used in type IV osteomyelitis. The defect is filled with a large PMMA spacer and the soft tissues closed over it. Over several weeks, a membrane forms around the PMMA spacer, which is probably osteoinductive. After 6 to 8 weeks, the spacer is removed, bone graft is packed into the defect, and the membrane is closed tightly around the graft. Over many months, the graft is incorporated and remodelled. Large segmental defects (>5 cm) are the most challenging group to manage. This technique has a high complication rate.[98] | |
Single-stage reconstruction | Becoming more common. For a large defect, a combination of a vascularised free fibula graft and an overlying free-muscle flap is an option. May be more appropriate for Class B hosts to reduce the number of operations. Common in children. | |
Ilizarov bone transport | A safer technique that requires sufficient remaining bone to allow the creation of a transport segment. Can be combined with free-muscle flaps, assuming the pin and wire placement will not impinge on the pedicle during transport. Allows immediate limb functioning.[99][100][101][102] Technically demanding and needs careful planning to ensure complications are minimised. |
Bone stabilisation
Instability must be addressed to cure infection. If a free flap is required, the frame must be planned preoperatively to give sufficient access to vessels for microvascular anastomosis. Antibiotic-coated implants for internal fixation are available but evidence supporting their efficacy and safety remains limited. Research into the use of antibiotic or antimicrobial coated intramedullary nails is ongoing. Exchange nailing for infected tibial non-unions is not recommended as it carries a high failure rate.[103]
Soft-tissue cover
In femoral, humeral, and spinal disease, primary closure of wounds is usually achievable.
In wounds around the pelvis, closure may be made easier by bone resection of the ilium or ischium.
Extensive pressure sores may prevent primary closure and a local flap may be required. Free-muscle flaps covered with split skin grafts are suitable because they provide a robust coverage of bone and are highly vascular and consequently able to support bone healing and supply systemic antibiotics to the soft tissues.[75][104]
If a soft tissue procedure is also required, the location of pin sites and metalwork should be agreed with the plastic surgeons.
Vacuum-assisted closure devices are of very limited use in osteomyelitis.
These should not be applied to sinuses with untreated bone infection as they unduly delay time to definitive surgery.
They may have a limited role in treating those with extensive soft-tissue defects following debridement who are not fit enough for free tissue transfer.
Antibiotic therapy in chronic osteomyelitis
The optimal duration of antimicrobial therapy after surgery is not certain. If the osteomyelitic bone has been fully resected, a shorter course of antibiotic therapy (2-6 weeks) may be adequate as long as operative wounds are healing without any signs of infection.
Long-term suppressive antibiotic therapy
In some cases where the patient is unfit for surgery or unwilling to have surgery, biopsy-guided or empirical antibiotic therapy may alleviate symptoms.
More studies are needed to determine the best antibiotic regimen and duration of therapy.
Long-term suppressive antibiotic therapy needs to be balanced against significant risk of poor adherence, adverse effects, drug interactions, and antimicrobial resistance.
Use of this content is subject to our disclaimer