Investigations
1st investigations to order
FBC with differential
Test
Required as part of the diagnostic work-up for HL.
Abnormal blood count may suggest bone marrow involvement.
Haemoglobin <10.5 g/dL is an adverse prognostic factor.
Result
low Hb and platelets; WBC count may be high or low
comprehensive metabolic panel
Test
Required as part of the diagnostic work-up for HL.
Performed to evaluate baseline liver and renal function prior to commencement of treatment.
Includes alkaline phosphatase, lactate dehydrogenase, liver enzymes, and albumin.
Low albumin (<4 g/dL) is an adverse prognostic factor.
Result
normal in most patients with HL
erythrocyte sedimentation rate (ESR)
Test
Required as part of the diagnostic work-up for HL.
ESR >50 mm/hour without B symptoms or >30 mm/hour with B symptoms is an adverse prognostic factor.
Result
may be elevated
thyroid function tests
Test
Baseline thyroid function tests are required, particularly in patients receiving radioherapy to the neck. Radiotherapy to the neck can increase the risk of developing thyroid dysfunction.
Result
may be normal at baseline and abnormal following radiotherapy to the neck
screening for HIV, hepatitis B, hepatitis C
Test
Infection with HIV, hepatitis B, and/or hepatitis C may complicate treatment.
Result
may be positive for HIV, hepatitis B, and/or hepatitis C
CXR
Test
Helpful at diagnosis to evaluate for bulky mediastinal lymphadenopathy.
Bulky mediastinal lymphadenopathy (exceeding one third of the intra-thoracic measurement on an upright posteroanterior film at the T5 to T6 inter-space) is an adverse prognostic factor.[Figure caption and citation for the preceding image starts]: CXR of patient presenting with dyspnoea, showing widened mediastinum and tracheal displacementFrom the personal collection of CR Kelsey [Citation ends].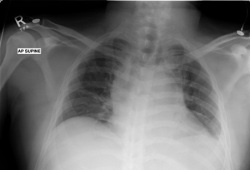
Result
mediastinal mass; bulky mediastinal lymphadenopathy
PET/CT scan
Test
PET/CT imaging (where available) should be carried out at diagnosis to evaluate the extent of disease (i.e., stage), and throughout treatment to monitor treatment response and guide management.[34][35]
A pre-treatment PET/CT is recommended to facilitate interpretation of post-treatment PET/CT scans.[36][37]
Patients treated with combined-modality therapy should undergo PET/CT imaging after chemotherapy to assess treatment response (e.g., based on the Deauville criteria) and to guide subsequent treatment (e.g., with chemotherapy and/or radiotherapy).[40]
PET appears to be an accurate staging tool. The sensitivity and specificity of PET are reported to be 93% and 87%, respectively.[41]
Patients adequately staged with PET/CT do not require a bone marrow biopsy to evaluate bone involvement.[38][Figure caption and citation for the preceding image starts]: PET scan (axial) showing fluorodeoxyglucose (FDG)-avid mass in the anterior mediastinumFrom the personal collection of CR Kelsey [Citation ends].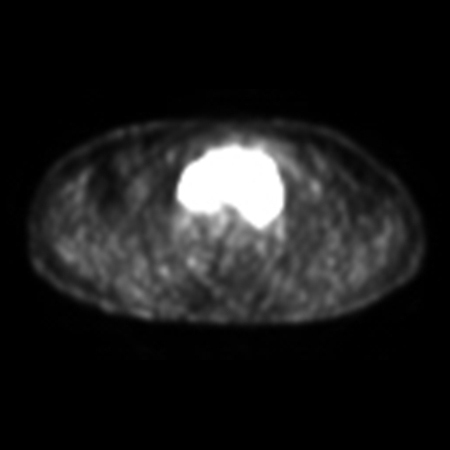 [Figure caption and citation for the preceding image starts]: PET scan (coronal) showing fluorodeoxyglucose (FDG)-avid mass in the superior mediastinumFrom the personal collection of CR Kelsey [Citation ends].
[Figure caption and citation for the preceding image starts]: PET scan (coronal) showing fluorodeoxyglucose (FDG)-avid mass in the superior mediastinumFrom the personal collection of CR Kelsey [Citation ends].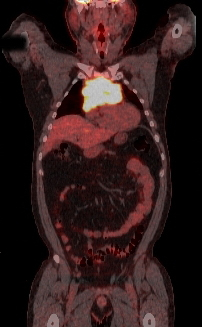
Result
involved sites appear FDG-avid (bright) with PET imaging
gallium scan
Test
A gallium scan can be used to assess the extent of disease if PET/CT is unavailable.
Gallium delivers a higher radiation dose to the patient than fluorodeoxyglucose (FDG)-PET.
Gallium scans appear to be less sensitive than FDG-PET scans, and it can take several days to obtain results.[42]
Gallium uptake in the bowel can obscure visualisation of abdominal disease.
Result
involved sites appear bright on gallium scans
contrast-enhanced CT (neck, chest, abdomen, pelvis)
Test
Indicated in combination with gallium scan when PET/CT scan is unavailable. [Figure caption and citation for the preceding image starts]: CT scan of patient with nodular sclerosis Hodgkin's lymphoma, showing an 11-cm anterior mediastinal massFrom the personal collection of CR Kelsey [Citation ends].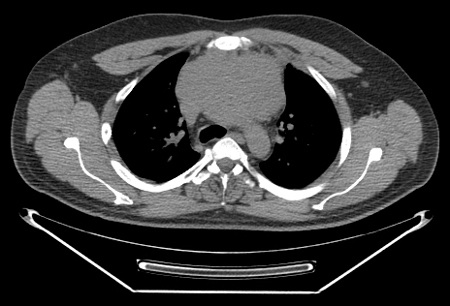
Result
may show enlarged lymph nodes and other sites of disease
excisional lymph node biopsy or core biopsy
Test
Required to confirm a diagnosis of HL, and essential before treatment can commence.
An excisional biopsy of an enlarged lymph node is recommended.[5][33] Under certain circumstances a core biopsy can be adequate.
A fine-needle aspiration biopsy is never suitable for diagnosing HL.
The Hodgkin's cell can be a characteristic Reed-Sternberg cell, or one of its variants, such as the lacunar cell in the nodular sclerosis sub-type. In nodular lymphocyte-predominant HL, the characteristic cell is the lymphocytic and histiocytic (L&H) cell, also referred to as a popcorn cell.[Figure caption and citation for the preceding image starts]: A diagnostic Reed-Sternberg cell is seen in the centre of the imageFrom the personal collection of CR Kelsey [Citation ends].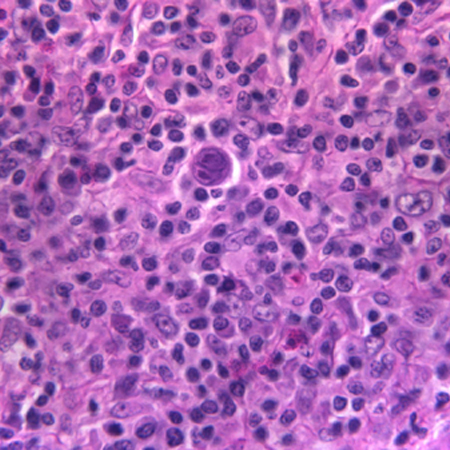
Result
Hodgkin's cells within an appropriate background cellular milieu
immunohistochemical studies
Test
Immunohistochemical studies are invaluable in differentiating HL from other lymphomas, as well as non-haematological processes.
Result
classical HL is characteristically CD30-positive and usually, but not always, CD15-positive. Typically, CD45 is negative, while CD20 positivity can be seen in up to 30% to 40% of classical HL. Nodular lymphocyte-predominant HL is usually CD15- and CD30-negative but CD20- and CD45-positive
Investigations to consider
bone marrow biopsy
echocardiogram or multi-gated acquisition (MUGA) scan
Test
Patients receiving potentially cardiotoxic chemotherapy agents (e.g., anthracyclines) should have a baseline cardiac evaluation.
Result
uneven distribution of technetium in the heart and/or decreased ejection fraction during a MUGA scan is indicative of heart disease; an echocardiogram can evaluate multiple cardiac parameters, including ejection fraction, which may be diminished in patients with heart disease
pulmonary function tests
Test
Patients receiving mediastinal radiotherapy and/or chemotherapy with potential for pulmonary toxicity (e.g., bleomycin) should have baseline pulmonary function tests.
FEV1 and diffusion capacity of lung for carbon monoxide are the most common parameters.
Result
may show decreased lung volumes, spirometry, or diffusion capacity in patients with lung disease related to smoking, or who have received pulmonary toxic chemotherapy or mediastinal radiotherapy
Use of this content is subject to our disclaimer