Recommendations
Key Recommendations
Have a high index of suspicion for acute aspiration and take prompt action if this is witnessed or suspected, particularly if the patient has a reduced conscious level. Note that management of acute aspiration and subsequent pneumonitis depends on the clinical status of the patient.
Place the patient semi-prone, tilt them into a 30° head-down position, and suction their oropharynx gently.
Give oxygen if needed. Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Other supportive measures may include endotracheal intubation and insertion of a nasogastric tube.
Organise bronchoscopy if you suspect the patient has aspirated a substantial amount of gastric content (>20-25 mL), or for any patient with barium aspiration. Get help from the critical care team if the patient has significant hypoxia or respiratory distress because bronchoscopy may cause further deterioration.
Unless the patient is receiving mechanical ventilation, do not give immediate antibiotic therapy routinely if the patient has aspiration pneumonitis, even if they have associated fever, leukocytosis, or pulmonary infiltrates.[2]
Consider a period of observation, depending on the patient’s clinical status and the underlying cause of acute aspiration.
Suspect aspiration pneumonia if the patient has non-resolving pneumonitis 48 hours after a confirmed or probable aspiration event. However, note that not all episodes of aspiration lead to an infection. See Aspiration pneumonia.
Have a high index of suspicion for acute aspiration and take prompt action if this is witnessed or suspected, particularly if the patient has a reduced conscious level. Note that management of acute aspiration and subsequent pneumonitis depends on the clinical status of the patient.
If you suspect acute aspiration, or this is witnessed, immediately place the patient semi-prone, and tilt them into a 30° head-down position.
This positions the patient’s larynx at a higher level than the oropharynx and allows the aspirated contents to drain externally.
Suction the patient’s oropharynx gently and take care to avoid initiating a gag reflex that may worsen aspiration.
Assess the patient’s oxygen requirements. Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[56]
A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[57]
Evidence: Target oxygen saturation in acutely ill adults
Too much supplemental oxygen increases mortality.
Evidence from a large systematic review and meta-analysis supports conservative/controlled oxygen therapy versus liberal oxygen therapy in acutely ill adults who are not at risk of hypercapnia.
Guidelines differ in their recommendations on target oxygen saturation in acutely unwell adults who are receiving supplemental oxygen.
The 2017 British Thoracic Society (BTS) guideline recommends a target SpO2 range of 94% to 98% for patients not at risk of hypercapnia, whereas the 2022 Thoracic Society of Australia and New Zealand (TSANZ) guideline recommends 92% to 96%.[57][58]
The 2022 Global Initiative For Asthma (GINA) guidelines recommend a target SpO2 range of 93% to 96% in the context of acute asthma exacerbations.[59]
One systematic review including a meta-analysis of data from 25 randomised controlled trials published in 2018 found that in adults with acute illness, liberal oxygen therapy (broadly equivalent to a target saturation >96%) is associated with higher mortality than conservative oxygen therapy (broadly equivalent to a target saturation ≤96%).[56] In-hospital mortality was 11 per 1000 higher for the liberal oxygen therapy group versus the conservative therapy group (95% CI 2 to 22 per 1000 more). Mortality at 30 days was also higher in the group who had received liberal oxygen (relative risk 1.14, 95% CI 1.01 to 1.29). The trials included adults with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery. Studies that were limited to people with chronic respiratory illness or psychiatric illness, or patients on extracorporeal life support, receiving hyperbaric oxygen therapy, or having elective surgery, were all excluded from the review.
An upper SpO2 limit of 96% is therefore reasonable when administering supplemental oxygen to patients with acute illness who are not at risk of hypercapnia. However, a higher target may be appropriate for some specific conditions (e.g., pneumothorax, carbon monoxide poisoning, cluster headache, or sickle cell crisis).[60]
In 2019 the BTS reviewed its guidance in response to this systematic review and meta-analysis and decided an interim update was not required.[61]
The committee noted that the systematic review supported the use of controlled oxygen therapy to a target.
While the systematic review showed an association between higher oxygen saturations and higher mortality, the BTS committee felt the review was not definitive on what the optimal target range should be. The suggested range of 94% to 96% in the review was based on the lower 95% confidence interval and the median baseline SpO2 from the liberal oxygen groups, along with the earlier 2015 TSANZ guideline recommendation.
Subsequently, experience during the COVID-19 pandemic has also made clinicians more aware of the feasibility of permissive hypoxaemia.[62]
Management of oxygen therapy in patients in intensive care is specialised and informed by further evidence (not covered in this summary) that is more specific to this setting.[63][64][65]
Once you have suctioned the patient’s oropharynx, and given oxygen (if required), ask for an immediate review from the critical care team to consider endotracheal intubation if the patient:
Is at risk of further aspiration
Is unable to protect their own airway (regurgitation, poor cough reflex)
Shows signs of respiratory failure (tachypnoea, dyspnoea, confusion, cyanosis).
Once the airway is secured, insert a nasogastric tube to empty the patient’s stomach and, where possible, tilt the patient to a 45° head-up position to help prevent further aspiration.
If the patient is intubated, positive-pressure ventilation with positive end-expiratory pressure can be used to protect the airway or to manage respiratory failure. However, always perform endotracheal suctioning before positive-pressure ventilation is used, to avoid forcing aspirated material deeper into the lungs.[11][16]
Do not give immediate antibiotic therapy routinely if the patient has aspiration pneumonitis, even if they have associated fever, leukocytosis, or pulmonary infiltrates.[2]
For patients who have aspirated gastric contents, note that gastric aspirate is sterile under normal conditions due to the low pH, so bacterial infection does not tend to occur in the early stages of acute lung injury.[2]
Immediate use of antibiotics may also select resistant organisms in uncomplicated chemical pneumonitis.
However, always give antibiotics if the patient is receiving mechanical ventilation; they are at high risk of developing ventilator-associated pneumonia. In addition, consider antibiotics if the patient has any of the following:
Gastroparesis (often seen in critically ill patients)
Small bowel obstruction
Possible colonisation of the stomach (e.g., patients taking proton-pump inhibitors, H2 antagonists, or antacids, where the stomach pH is less acidic)
Aspiration pneumonitis that does not resolve within 48 hours. See Non-resolving pneumonitis after 48 hours: suspected aspiration pneumonia below.
Check your local protocol for choice of antibiotic, and always obtain respiratory cultures before starting antibiotics. Stop or modify the antibiotics within 72 hours based on the culture results.[1]
More info: Corticosteroids
Human studies have shown no improvement in mortality, and the rate of gram-negative pneumonia 5 days after aspiration was higher in patients receiving corticosteroids.[66] Although the infiltrates improve more quickly in the patients given corticosteroids than in those given placebo, patients given corticosteroids might have a longer intensive care unit (ICU) stay.[67][68]
Overall, because of the increased risk for gram-negative bacterial pneumonia and a prolonged stay in the ICU, together with the lack of any mortality benefit, corticosteroids are not indicated for aspiration pneumonitis. They are also not indicated with aspiration pneumonitis that is complicated by acute respiratory distress syndrome.[2]
If you suspect a substantial amount of gastric content (>20-25 mL) is likely to have been aspirated, organise urgent (within a few hours) bronchoscopy and suctioning to remove any aspirated gastric fluid and solid material from the central airways.[69]
This helps to reduce inflammatory reaction, prevent lung collapse, and lower the risk of subsequent infection.
More info: Substantial aspiration of gastric contents
A volume of gastric aspirate >0.3 mL per kilogram of body weight (i.e., 20-25 mL) with a pH <2.5 is deemed necessary for the development of aspiration pneumonitis, although aspiration of particulate food matter can cause severe pulmonary damage, even if the pH of the aspirate is >2.5.[2] Animal studies have shown a biphasic pattern to injury, with an initial peak at 1 to 2 hours after aspiration (direct burn effects) and a second peak at 4 to 6 hours (related to neutrophil infiltration).[2]
Organise early bronchoscopy if you suspect the patient has aspirated barium during gastrointestinal studies, to remove the barium from their airway, and reduce hypoxaemia.
Get help from the critical care team if the patient has significant hypoxia or respiratory distress because bronchoscopy may cause further deterioration.
Other indications for bronchoscopy are to:
Clear the airway if the aspirated material is particulate or if there is radiographic evidence of lobar or segmental collapse
Collect quantitative cultures on bronchoalveolar lavage or protected specimen brush, which can be used to guide antibiotic therapy, particularly in patients who do not respond to empirical antibiotic treatment
Investigate alternative diagnoses that can cause a similar radiographic pattern to acute aspiration. These include acute respiratory distress syndrome imitators such as COVID-19 pneumonitis/pneumonia, acute interstitial pneumonitis (Hamman-Rich syndrome), acute eosinophilic pneumonia, cryptogenic organising pneumonia, diffuse alveolar haemorrhage, and acute hypersensitivity pneumonitis.[41]
[Figure caption and citation for the preceding image starts]: Bronchoscopy showing barium aspiration in a lung transplant patient in the right mainstem bronchus after a barium swallow studyFrom the collection of Dr Kamran Mahmood [Citation ends].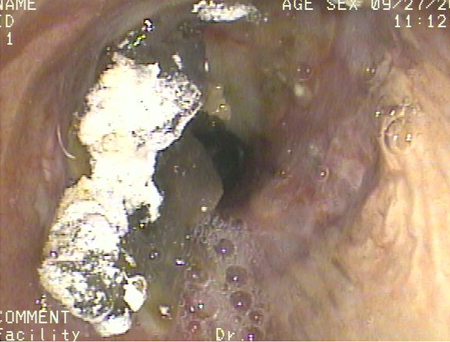
Consider observing the patient closely in hospital or in another care facility for at least 48 hours following acute aspiration, depending on their clinical status and the underlying cause of aspiration. The patient will often have a reduced level of consciousness following aspiration.
If the patient has aspirated barium, severe long-term harm is unlikely in most patients due to the inert nature of barium sulfate. However, consider admission for observation (if the patient isn’t already in hospital) for older patients, and those with significant symptoms; they are at increased risk of severe pneumonitis and death.[8]
Involve the multidisciplinary team early for any patient with suspected dysphagia and organise a swallowing assessment. Always involve the patient and/or carer in decision-making where possible.[50][51] See Assessment of dysphagia.
An initial screen of swallowing function should be completed by an appropriately trained healthcare professional.[52][53] Screening questions that may be used are:[54]
Do you cough and/or choke when you eat and drink?
Do you ever feel like food or drink goes down ‘the wrong way?’
Does it take you longer to eat your meals than it used to?
Have you changed the type of food that you eat?
Does your voice change after eating/drinking?
Discuss keeping the patient 'nil by mouth' with a senior colleague or speech and language therapist if the initial swallowing assessment indicates dysphagia.
In addition, organise a specialist swallowing assessment (e.g., by a speech and language therapist).[50][52] See Screening.
Give food, fluids, and medication in a form that is appropriate for your patient once they have had a swallowing assessment.[50][52]
Consider alternative feeding strategies if the patient is unable to take adequate food, fluids, and medication orally.[52] Tube feeding (e.g., nasogastric tube, gastrostomy, or nasal bridle tube) may be appropriate to provide temporary nutritional support for patients with non-progressive causes of dysphagia such as stroke.[52]
Ensure the patient is screened for malnutrition and dehydration and involve a dietician to optimise the patient’s nutritional needs.[52]
Discuss feeding strategies carefully with the patient and/or family because the risk of aspiration may be outweighed by the patient’s quality-of-life needs, especially in progressive disease. It may be preferable for the patient to eat and drink (while accepting it is unsafe) rather than using a modified diet, feeding tube, or 'nil by mouth' regimens.[51] This strategy is also known as 'risk feeding'.[70]
Practical tip
Be aware that thickened fluids can alter the pharmacokinetics of the patient’s medication by reducing the bioavailability.[71] Seek advice from a senior colleague or pharmacist.
Other management strategies include swallowing rehabilitation, education, careful positioning when feeding, and referral to an oral hygienist/dentist.[50]
If a patient does not have a known cause for their dysphagia (e.g., not known oropharyngeal dysphagia), organise urgent referral for upper gastrointestinal endoscopy (to be performed within 2 weeks) to assess for upper gastrointestinal cancer.[52]
Suspect aspiration pneumonia if the patient has non-resolving pneumonitis 48 hours after a confirmed or probable aspiration event.
Clinical features of aspiration pneumonia include cough, breathlessness, fever, and persistent leukocytosis.
Order a repeat chest x-ray if you suspect aspiration pneumonia clinically; confirm the diagnosis if infiltrates are present >48 hours after a confirmed or suspected aspiration event.
Pneumonia can also occur due to tissue damage secondary to pneumonitis rather than due directly to the aspiration event.
Start empirical antibiotics to treat the infection and prevent complications such as empyema and lung abscess formation.
Always send a sputum sample for Gram stain and culture where possible before starting antibiotics.
Empirical antibiotic treatment for aspiration pneumonia is the same as that for non-aspiration pneumonia (community-acquired, hospital-acquired, or ventilator-associated), unless the patient has anaerobic pleuropulmonary syndrome (a later presentation of cavitary pneumonia or empyema associated with prior loss of consciousness and poor dental hygiene). See Community-acquired pneumonia (non-COVID-19) and Hospital-acquired pneumonia (non-COVID-19).
Empirical treatment for aspiration pneumonia does not require coverage for anaerobic organisms.[72] Similarly, no additional anaerobic antibiotic coverage is warranted for patients with dysphagia or aspiration associated with stroke.[73]
Most patients with aspiration pneumonia are treated initially with intravenous antibiotics.
Seek advice from a microbiologist on selection of antibiotic treatment and consider local and ward-based resistance data. Follow your local protocol.
Patients with aspiration pneumonia will need additional further management. See Aspiration pneumonia.
Use of this content is subject to our disclaimer