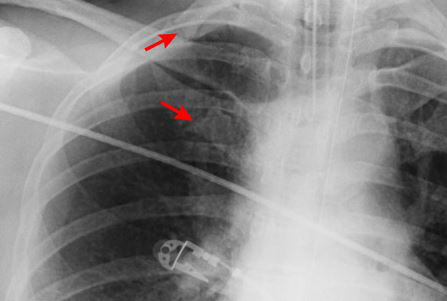
Summary
Definition
History and exam
Risk factors
- blunt chest trauma
- cardiopulmonary resuscitation (CPR)
- physical abuse in children
- osteoporosis
- age >65 years
- participation in sport
- primary bone tumours
- metastatic bone tumours
- severe cough
Diagnostic investigations
Investigations to consider
- CT of head, cervical spine, abdomen, and pelvis
- echocardiography
- skeletal survey and CT head (children)
Treatment algorithm
Contributors
Expert advisers
Michael Barrett, MBChB, FRCS (Tr & Orth), PG Cert Med Ed
Consultant Trauma and Orthopaedic Surgeon
Cambridge University Hospitals NHS Foundation Trust
Cambridge
UK
Disclosures
MB is a director of Orthohub.xyz, an online education platform for orthopaedic surgeons. Orthohub.xyz receives sponsorship from the healthcare industry.
Acknowledgements
BMJ Best Practice would like to gratefully acknowledge the previous expert contributors, whose work has been retained in parts of the content:
Peter Cundy, MBBS, FRACS
Associate Professor
Discipline of Orthopaedics & Trauma
University of Adelaide
Paediatric Orthopaedic Surgeon
Women's and Children's Hospital
Adelaide
Australia
Nicole Williams, BMedSc (hons I), BMed, Grad Cert Sports Med, FRACS (Ortho)
Associate Professor
Orthopaedics and Trauma
University of Adelaide
Head of Orthopaedic Surgery and Major Trauma Service Co-Director
Women's and Children's Hospital
Adelaide
Australia
Disclosures
PC is on the boards of MIGA (medical indemnity insurance) and Orthopaedics SA. He owns stock though a personal superannuation fund. PC’s hospital, the Women's & Children's Hospital, Adelaide, receives institutional support from medical supply companies for salary of research scientists. PC received payment from a medical supplier for two lectures on wound dressings in 2017. PC declares that none of the above are relevant to the Rib fractures topic and that he receives no other money from suppliers. NW’s hospital, the Women's & Children's Hospital, Adelaide, receives institutional research support from medical supply companies and BioMarin Pharmaceutical Inc.
Peer reviewers
Douglas West, BSc(Hons), MB, ChB, FRCS(CTh), Dip Epidemiol (Lond)
Consultant Thoracic Surgeon
University Hospitals Bristol and Weston NHS Foundation Trust
Bristol
UK
Biography
DW is Thoracic Surgery Audit Lead and Audit Committee Co-Chair, the Society for Cardiothoracic Surgery; Cardiothoracic National Clinical Lead, Getting it Right First Time (GIRFT), NHS England Improvement; Board Member, Cardiothoracic Specialty Specific Group, the Royal College of Surgeons of Edinburgh. DW is Co-Chair, Lung Cancer Guideline Update Committee, NICE (2019) and Member, Lung Cancer Quality Standards Committee, NICE (2019). DW has been a member of the NHS England Clinical Expert Group on Lung Cancer.
Disclosures
DW has received speaker’s fees from AstraZeneca for events related to lung cancer care; research grants and speaker’s fees from Medtronic, a medical devices company; speaker’s fees and travel expenses from Ethicon, a medical devices company, for events related to training in thoracic and spinal surgery; and reimbursement of travel expenses from Bristol Myers Squibb for events related to lung cancer. DW has edited patient education materials (unpaid) for the Roy Castle Foundation, and has been reimbursed by it for travel expenses incurred attending meetings of the NHS England Clinical Expert Group in Lung Cancer.
Use of this content is subject to our disclaimer