Complications
Rates of abdominal compartment syndrome (ACS) are estimated at approximately 20% after open repair of ruptured AAA.[3][212] A small retrospective review reported ACS in 34% of patients following open repair of ruptured AAA and 21% after endovascular aneurysm repair (EVAR).[213] A nationwide population-based study (Swedish Vascular Registry) found that after ruptured AAA repair, ACS developed in 6.8% following open repair compared with 6.9% after EVAR.[214] A systematic review of 46 studies, including 3064 patients, showed that ACS affects approximately 9% of patients following EVAR for ruptured AAA.[215] ACS is associated with significant increases in perioperative mortality.[215]
Assess people for abdominal compartment syndrome if their condition does not improve after EVAR or open surgical repair of a ruptured AAA.[42] Patients with abdominal compartment syndrome after open or endovascular treatment of a ruptured AAA should be treated with decompressive laparotomy.[3]
Ileus has been reported in 11% of patients, with intestinal obstruction and colitis each occurring in 1% of patients undergoing open repair.[193] One review reported a prevalence of clinically relevant bowel ischaemia of approximately 10% following surgery for ruptured AAA.[216] If colonic ischaemia is suspected in patients undergoing open or endovascular treatment for AAA, flexible sigmoidoscopy should be considered to confirm the diagnosis.[3] Ischaemic colitis requiring colectomy is rare.
Following EVAR and open repair of AAA, there is a significant incidence of acute kidney injury (AKI).[217][218][219] In open repair, this seems to be transient. However, following EVAR the causes are multifactorial, and decline in renal function is significantly greater (especially with suprarenal fixation) than in open surgery.[220] AKI following EVAR is associated with medium-term increased morbidity and mortality.[217] A strategy to preserve renal function by dose reduction of iodine contrast media, withdrawal of nephrotoxic drugs, and ensuring adequate hydration should be considered in patients undergoing EVAR of a complex AAA.[3] Preservation of large accessory renal arteries (4 mm) should also be considered.[3] Data from small randomised controlled trials support the potential use of several interventions, including mannitol, antioxidant supplements, an extraperitoneal approach for open repairs, and human atrial natriuretic peptide, to reduce the incidence of AKI after elective AAA repair.[221] However, larger multicentre randomised trials of higher quality will help determine which interventions are effective.
Post-implantation syndrome, a poorly understood complication of EVAR, occurs in the early postoperative period and may last for up to 10 days following EVAR.[222] Fever, malaise, and back pain, which may be due to cytokine release, are typical. Early major cardiovascular events are more common in patients who develop post-implantation syndrome, although there do not appear to be any long-term implications or impact on mortality.[222][223]
Rates of amputation due to limb ischaemia were very low (0.1% at 30 days) in a large series of patients (n=1135) who underwent open repair.[193] Patients with new-onset or worsening of lower limb ischaemia should be evaluated immediately for graft-related problems, such as limb kinking or occlusion.[3]
Spinal cord ischaemia is rare after EVAR, with an incidence in the EUROSTAR collaborators registry of 0.21%.[224] In a retrospective analysis of emergency endovascular treatment for ruptured AAA, 4 of 35 patients (11.5%) developed spinal cord ischaemia postoperatively.[225] Delayed spinal cord ischaemia (developing 2 days after EVAR) has been reported.[226] Increased operative time, intravascular handling, and difficult anatomy all contribute to an increased risk of spinal cord ischaemia.[227] Early recognition is essential, with treatment including spinal drains and administration of steroids. Only 25% of cases will recover, with 25% showing some improvement, and 50% no improvement.[227]
Damage to the autonomic nerves (present at the aorto-iliac bifurcation) during dissection in open repair, as well as reduction in pelvic blood supply, can result in impotence and retrograde ejaculation. EVAR can also result in significant erectile dysfunction, mainly due to internal iliac artery (IIA) occlusion. Studies comparing rates of erectile dysfunction after open repair versus EVAR have shown inconsistent findings. One review estimated incidence of new erectile dysfunction of between 20% and 83% in the first year after open repair (variation depended on trial type).[228] The estimated incidence of new erectile dysfunction was no greater than 14.3% following endovascular repair (and was worse after bilateral rather than unilateral IIA occlusion).[228] However, a prospective single-centre study found no statistically significant difference in de novo erectile dysfunction between open repair and EVAR groups.[229] Approximately 27% of the patients reported erectile dysfunction before open repair, increasing to 53% 1 year after surgery. The corresponding frequencies after EVAR were 43% and 59%, respectively.[3][229] For patients treated for AAA who are distressed by postoperative new-onset sexual dysfunction, referral to specialised teams should be considered.[3]
One case series reported para-anastomotic aneurysms in 10% of patients after aortic bypass grafting.[230] The rate of femoral anastomotic pseudoaneurysm may be as high as 20% at 10 years after aortobifemoral reconstruction for AAA.[231] For patients with para-anastomotic aneurysm formation, infection as underlying cause should be considered.[3] Graft infection may be the underlying cause of secondary aneurysm formation, particularly within the first years after repair.[3] For patients with non-infectious aorto-iliac para-anastomotic aneurysm formation after previous abdominal aortic aneurysm repair, endovascular repair should be considered preferentially.[3][232]
Aortic neck dilation occurred in 24.6% of EVAR patients during 15 months to 9 years of follow-up.[153] A composite clinical event of endoleak, migration, and re-intervention was significantly more common in this group than in those patients without aortic neck dilation.
Can result from infection during implantation, or haematogenous seeding following dental procedures or endoscopic procedures with biopsy. Incidence is low: a retrospective cohort study found that the 2-year rate of graft infection was 0.19% following open repair versus 0.16% with EVAR.[233] Surgical removal of the infected endograft is the optimum treatment; there is a high mortality rate with medical management alone.[3][234]
Ureteric obstruction is related to encasement of the ureters in an inflammatory perianeurysmal fibrosis of unresolved aetiology rather than secondary to aneurysm compression.[235] Most often, ureteral compression is associated with inflammatory aortic aneurysm. Extensive retroperitoneal adhesions may result in ureteral obstruction in 18% of patients. The inferior vena cava may become involved.[236]
Duodenal obstruction is a consequence of compression of the duodenum in its fixed retroperitoneal course between the aneurysmal aorta and the superior mesenteric artery.[235]
The incidence of graft limb occlusion up to 10 years after open AAA repair has been reported as being between 2.6% and 3.0%.[237][238] The risk of graft occlusion is greater with EVAR, with a reported incidence of up to 7.2% in follow-up studies.[239] Kinking is a risk factor for graft limb occlusion following EVAR.[240]
Endoleak is persistent blood flow outside the graft and within the aneurysm sac after EVAR.[241][242] It is not a complication following open surgical repair.
Postoperative surveillance can detect major endoleaks and aneurysm sac expansion.
Order contrast-enhanced CT angiography if endoleak is suspected. Use contrast-enhanced ultrasound if contrast-enhanced CT is contraindicated.[42]
Risk of endoleak following EVAR is 24%.[241] There are five types of endoleak.
Type I:
Leak at the attachment site (type IA at the proximal end of the endograft or iliac occluder; type IB at the distal end); usually immediate, but delayed leaks may occur.[Figure caption and citation for the preceding image starts]: Type I endoleak at the distal left iliac anastomosis (leak encircled)University of Michigan, specifically the cases of Dr Upchurch reflecting the Departments of Vascular Surgery and Radiology [Citation ends].
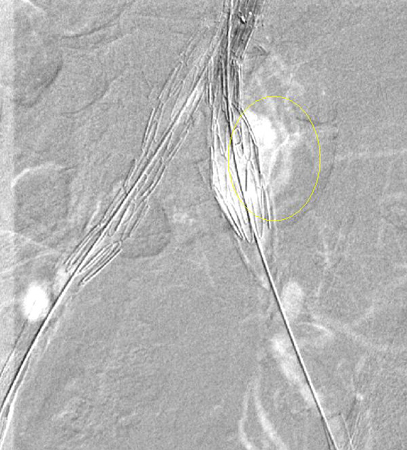
Every effort should be made to repair type I endoleak before completing the procedure (e.g., balloon moulding of the proximal seal zone, placement of a proximal cuff, endostaples, liquid embolisation).[243] For patients with a compromised proximal seal after endovascular AAA repair, proximal extension with fenestrated and branched devices should be considered.[3] Persistent type IA endoleak may necessitate conversion to open repair, provided the surgical risk is acceptable.[3][76][244][Figure caption and citation for the preceding image starts]: Extension stent graft deployed for the same type I endoleak (encircled)University of Michigan, specifically the cases of Dr Upchurch reflecting the Departments of Vascular Surgery and Radiology [Citation ends].
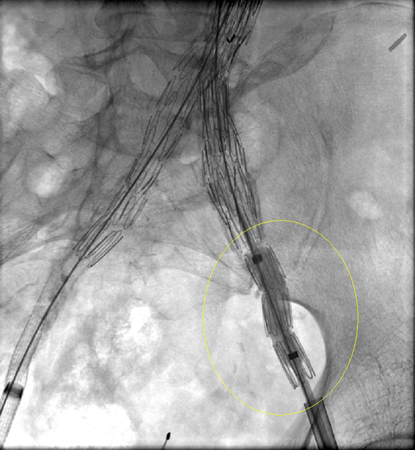 [Figure caption and citation for the preceding image starts]: Resolution of the type I endoleak resolved after extension deployedUniversity of Michigan, specifically the cases of Dr Upchurch reflecting the Departments of Vascular Surgery and Radiology [Citation ends].
[Figure caption and citation for the preceding image starts]: Resolution of the type I endoleak resolved after extension deployedUniversity of Michigan, specifically the cases of Dr Upchurch reflecting the Departments of Vascular Surgery and Radiology [Citation ends].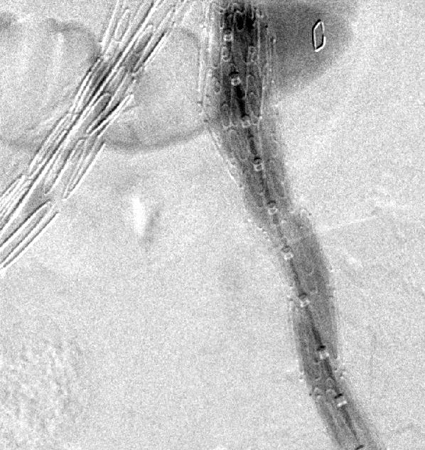
Type II:
Patent branch leak.[Figure caption and citation for the preceding image starts]: Type II endoleak (encircled) discovered on follow-up CTUniversity of Michigan, specifically the cases of Dr Upchurch reflecting the Departments of Vascular Surgery and Radiology [Citation ends].
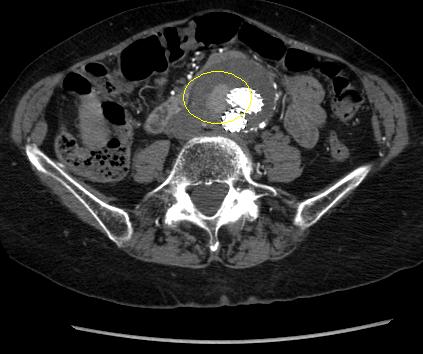
Spontaneous resolution may occur, although persistence may result in sac growth.[245]
If a type II endoleak or other abnormality of concern is observed on contrast-enhanced CT imaging at 1 month after EVAR, postoperative imaging at 6 months is recommended.[76] Around 50% of type II endoleaks are diagnosed before 30-day follow-up, 40% are diagnosed after 30 days of follow-up, and 8% are diagnosed after 12 months of follow-up.[246]
Treatment should only be considered in the presence of significant aneurysm sac growth (≥10 mm compared with baseline or with the smallest diameter during follow-up using the same imaging modality and measurement method), primarily by endovascular means, provided alternative causes including type I or III endoleaks have been excluded.[3]
Treatment of choice is transarterial coil embolisation, although laparoscopic ligation of collateral branches, direct percutaneous translumbar puncture of the sac, translumbar embolisation, and transcatheter transcaval embolisation have been reported.[242][247][248][249][250][251][252][253][254][255][256][257][258]
Type III:
Graft defect with leak through fabric tears, graft disconnection, or disintegration of the fabric.[241][242][259]
Repair is indicated upon discovery (endovascular stent graft extension).[3][76][248][260]
Type IV:
Type V (endotension):
Endotension is increased intrasac pressure after EVAR without visualised endoleak on delayed contrast CT scans.
Endotension is less common with the newer-generation grafts.[76]
There is no standardised method to measure endotension or consensus on indicated therapy in the absence of aneurysm enlargement; however, treatment of endotension to prevent aneurysm rupture is suggested in selected patients with continued aneurysm expansion.[76][242]
Incidence is 3% to 29%, most commonly affecting the digits (blue toe syndrome). There is a 5% incidence of distal embolisation resulting in limb-threatening ischaemia, digital ischaemia, and calf myonecrosis.[261]
Patients with aneurysmal disease have a high prevalence of abdominal wall hernias, and 11% to 37% have postoperative incisional hernias following abdominal surgery.[262] Mesh augmentation during closure of the incision for open AAA repair can help reduce the occurrence of incisional hernias.[3][262]
Use of this content is subject to our disclaimer