History and exam
Key diagnostic factors
common
altered pigmented lesion (ABCDE signs)
A melanocytic lesion that is changing with regard to size, shape, or colour is of concern.[Figure caption and citation for the preceding image starts]: Superficial spreading melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].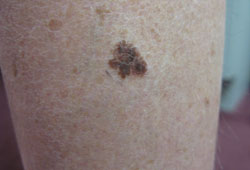 [Figure caption and citation for the preceding image starts]: Nodular melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].
[Figure caption and citation for the preceding image starts]: Nodular melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends]. The American Cancer Society ABCD signs of melanoma mnemonic is useful for both physicians and patients in the early detection of melanoma.[52] The addition of evolving (letter E added to the ABCD mnemonic) has been proposed to increase the sensitivity and specificity of diagnosis using the ABCD rule.[50]
The American Cancer Society ABCD signs of melanoma mnemonic is useful for both physicians and patients in the early detection of melanoma.[52] The addition of evolving (letter E added to the ABCD mnemonic) has been proposed to increase the sensitivity and specificity of diagnosis using the ABCD rule.[50]
The mnemonic stands for: Asymmetry of the lesion, Border irregularity, Colour variability, Diameter >6 mm, Evolution.
melanocytic lesion that does not resemble surrounding melanocytic naevi ('ugly duckling')
uncommon
spontaneous bleeding or ulceration of a pigmented lesion
A melanocytic lesion that spontaneously bleeds or ulcerates is concerning for melanoma.
constitutional symptoms
Weight loss, fatigue, night sweats, headache, or cough may be symptoms of a late presentation (with metastatic disease) or progression in a patient with a history of melanoma.
nail sign: persistent single-nail melanonychia striata
A persistent pigmented band in a single nail in an adult that is >5 mm in diameter and without a definite cause, such as medicine-induced pigmentation or trauma, is concerning for melanoma of the nail apparatus.[77][Figure caption and citation for the preceding image starts]: Subungual melanoma in situFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].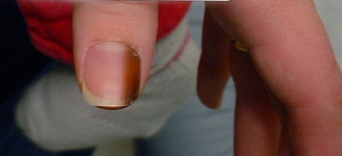
nail sign: Hutchinson's sign
In the setting of pigmented bands in the nail bed and matrix (melanonychia striata), this sign shows extension of pigment into the proximal or lateral nail fold.
atypical dermoscopy findings
Bluish-white veiled appearance within a melanocytic lesion corresponds to dermal fibrosis, with pigmented melanophages in the dermis on histological examination of the pigmented lesion. These features represent regression and are common features of melanoma.[Figure caption and citation for the preceding image starts]: Bluish-white veil of a melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].
Dermoscopic findings may also include atypical globules and dots of different sizes and shapes, patches of atypical network, diffuse polymorphous vasculature, superficial spreading melanoma with pseudopods distributed asymmetrically around the lesion.
fixed lymphadenopathy
Fixed lymphadenopathy in a patient with a history of melanoma, especially in lymph node basins draining the site of melanoma excision, is concerning for metastasis.
in-transit metastases
Subcutaneous masses between the primary melanoma site and the draining lymph node basins should raise suspicion of in-transit metastases.
Risk factors
strong
sun exposure
Melanoma incidence increases with residence in, duration of residence in, and migration to, areas of high ambient solar radiation.[33] It is most common in people with Fitzpatrick type I (white) skin of European origin. Evidence suggests that the risk of melanoma increases with age due to the cumulative effects of sun exposure.[34]
Melanoma is most common at body sites that have received intermittent sun exposure (in particular, a history of intermittent intense exposure as a child). Such sites include the back in men and the legs in women.[35] Melanoma is also common in people with predominantly indoor occupations whose sun exposure is limited to weekends and vacations. Increased frequency or severity of sunburn increases the risk of melanoma.[36]
family history of melanoma
Risk for individuals with a family history of melanoma has been estimated to be 1.74 (relative risk; 95% CI 1.41-2.14).[17] Among children of a parent with multiple myelomas, a standardised incidence ratio of 61.78 (95% CI 5.82 to 227.19) has been reported.[37]
Mutations in the CDKN2A gene on chromosome 9 have been found in families with the atypical or dysplastic naevus syndrome and in 20% of melanoma-prone families.[18][38]
personal history of melanoma
The relative risk of developing a subsequent melanoma after any first melanoma (measured as standardised incidence ratio) varies from 12.4 (invasive after invasive melanoma; 95% CI 11.6 to 13.2) to 26.4 (in situ after in situ melanoma; 95% CI 22.6 to 30.7) compared with the general population.[39] At 20-year follow-up, approximately 5% of patients with a first invasive melanoma had developed a second invasive melanoma.
personal history of skin cancer (including actinic damage)
Presence of actinic damage (premalignant and skin cancer lesions) increases the risk for melanoma (relative risk 4.28; 95% CI 2.80-6.55).[17]
history of atypical naevi
Patients with multiple naevi that are 6 mm or larger with irregular pigmentation, irregular ill-defined borders, and central papular component with peripheral macular pigmentation are at increased risk, especially if the naevi occur in families. The risk of melanoma in such families is approximately 10%, and 100% if a history of a previous melanoma is present.[40]
One meta-analysis of case-control studies found that the relative risk of melanoma is 1.45 in patients with one atypical mole compared with those with no atypical moles, and this increases to 6.36 in those with five atypical moles.[41]
Fitzpatrick skin type I or II (white skin)
Fitzpatrick type I or II (white) skin (burns easily) increases the risk of melanoma compared with Fitzpatrick type V or IV (brown/black) skin (relative risk 2.06; 95% CI 1.68-2.52).[17]
red or blond hair colour
People with red hair (relative risk 3.64; 95% CI 2.56-5.37) or blond hair (relative risk 1.96; CI 1.41-2.74) are at elevated risk of melanoma compared with people with brown/black hair.[17]
high freckle density
High density of freckles is associated with increased melanoma risk (relative risk 2.10; 95% CI 1.80-2.45).[17]
sun bed use
Compared with never using, the odds ratio for melanoma associated with ever using indoor tanning beds was 1.16 (95% CI 1.05-1.28). Increased exposure (>10 tanning sessions) is associated with greater risk (odds ratio 1.34, 95% CI 1.05-1.71).[15]
light eye colour
increased numbers of benign-appearing melanocytic naevi
large congenital naevi
immunosuppression
xeroderma pigmentosum
Genetic syndrome with skin cancer predisposition.[47]
Use of this content is subject to our disclaimer