Diet and exercise is the initial approach for the obese patient who wishes to lose weight.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
[101]Shaw K, Gennat H, O'Rourke P, et al. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD003817.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003817.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/17054187?tool=bestpractice.com
[  ]
What are the effects of exercise in people who are overweight or obese?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.79/fullShow me the answer A combination of a reduced-calorie diet and exercise is more efficacious than either alone.[102]Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755-67.
http://www.ncbi.nlm.nih.gov/pubmed/17904936?tool=bestpractice.com
Additional weight loss may be possible with some medicine regimens.[102]Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755-67.
http://www.ncbi.nlm.nih.gov/pubmed/17904936?tool=bestpractice.com
The initial goal of weight loss therapy (diet and exercise) is a 10% reduction in body weight over a 6-month period.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
This equates to a loss of 0.23 to 0.454 kg per week in the patient with a BMI of ≤35 kg/m², and 0.454 to 0.91 kg per week in the patient with a BMI of >35 kg/m². After the initial 6-month period, the patient is re-assessed to determine the efficacy of the therapy, whether the patient needs to lose more weight, or whether a weight-maintenance programme may be established.
]
What are the effects of exercise in people who are overweight or obese?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.79/fullShow me the answer A combination of a reduced-calorie diet and exercise is more efficacious than either alone.[102]Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755-67.
http://www.ncbi.nlm.nih.gov/pubmed/17904936?tool=bestpractice.com
Additional weight loss may be possible with some medicine regimens.[102]Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755-67.
http://www.ncbi.nlm.nih.gov/pubmed/17904936?tool=bestpractice.com
The initial goal of weight loss therapy (diet and exercise) is a 10% reduction in body weight over a 6-month period.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
This equates to a loss of 0.23 to 0.454 kg per week in the patient with a BMI of ≤35 kg/m², and 0.454 to 0.91 kg per week in the patient with a BMI of >35 kg/m². After the initial 6-month period, the patient is re-assessed to determine the efficacy of the therapy, whether the patient needs to lose more weight, or whether a weight-maintenance programme may be established.
Dietary changes
For weight reduction, an intake of 1000-1200 kcal/day for women and 1200-1500 kcal/day for men should produce a caloric deficit of 500-1000 kcal/day.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
The main focus of dieting has been, and remains, a reduction in caloric intake.[103]Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009 Feb 26;360(9):859-73.
https://www.nejm.org/doi/10.1056/NEJMoa0804748
http://www.ncbi.nlm.nih.gov/pubmed/19246357?tool=bestpractice.com
Traditionally, the most important aspect of diet and exercise was to ensure that the caloric intake was less than the caloric expenditure, thus producing a caloric deficit.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
The most commonly recommended diet was the low-fat type. The evidence that dietary composition has an effect on weight loss independent of a reduction in total calories; however, is mixed. There is limited evidence that low-glycaemic and low-carbohydrate diets are both effective, but no single diet has emerged as superior to the others over the long term (i.e., >1 year).[73]Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007 May 16;297(19):2092-102.
https://jamanetwork.com/journals/jama/fullarticle/207088
http://www.ncbi.nlm.nih.gov/pubmed/17507345?tool=bestpractice.com
[103]Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009 Feb 26;360(9):859-73.
https://www.nejm.org/doi/10.1056/NEJMoa0804748
http://www.ncbi.nlm.nih.gov/pubmed/19246357?tool=bestpractice.com
[104]Shai I, Schwarzfuchs D, Henkin Y, et al; Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008 Jul 17;359(3):229-41.
https://www.nejm.org/doi/full/10.1056/NEJMoa0708681
http://www.ncbi.nlm.nih.gov/pubmed/18635428?tool=bestpractice.com
[105]Hession M, Rolland C, Kulkarni U, et al. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009 Jan;10(1):36-50.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1467-789X.2008.00518.x
http://www.ncbi.nlm.nih.gov/pubmed/18700873?tool=bestpractice.com
[106]Thomas DE, Elliott E, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD005105.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005105.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/17636786?tool=bestpractice.com
[107]Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003 May 22;348(21):2074-81.
https://www.nejm.org/doi/full/10.1056/NEJMoa022637
http://www.ncbi.nlm.nih.gov/pubmed/12761364?tool=bestpractice.com
[108]Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003 May 22;348(21):2082-90.
https://www.nejm.org/doi/full/10.1056/NEJMoa022207
http://www.ncbi.nlm.nih.gov/pubmed/12761365?tool=bestpractice.com
[109]Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015 Apr 7;162(7):501-12.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4446719
http://www.ncbi.nlm.nih.gov/pubmed/25844997?tool=bestpractice.com
[110]Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014 Sep 3;312(9):923-33.
https://jamanetwork.com/journals/jama/fullarticle/1900510
http://www.ncbi.nlm.nih.gov/pubmed/25182101?tool=bestpractice.com
[  ]
What are the effects of low glycemic index (GI) or low glycemic load (GL) diets for people with overweight or obesity?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4359/fullShow me the answer
]
What are the effects of low glycemic index (GI) or low glycemic load (GL) diets for people with overweight or obesity?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4359/fullShow me the answer
Some studies have found that the low-carbohydrate/high-protein diet produces greater weight loss than the low-fat diet at 6-month follow-up, and patients seem to prefer the low-carbohydrate/high-protein diet.[105]Hession M, Rolland C, Kulkarni U, et al. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009 Jan;10(1):36-50.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1467-789X.2008.00518.x
http://www.ncbi.nlm.nih.gov/pubmed/18700873?tool=bestpractice.com
[106]Thomas DE, Elliott E, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD005105.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005105.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/17636786?tool=bestpractice.com
One meta-analysis looked at results from patients adhering to 14 popular diets, categorised as low-carbohydrate, low-fat, and moderate-macronutrient, and analysed reduction in weight and cardiovascular outcomes at 6 months and 1 year. All three diet patterns resulted in weight loss and decreased blood pressure at 6 months compared with a regular diet; however, these effects largely disappeared after 1 year of dietary adherence.[111]Ge L, Sadeghirad B, Ball GDC, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. 2020 Apr 1;369:m696.
https://www.doi.org/10.1136/bmj.m696
http://www.ncbi.nlm.nih.gov/pubmed/32238384?tool=bestpractice.com
Adherence to the diet (i.e., compliance) and the reliability of patient reporting of caloric intake have been problematic in studies on dietary intervention. The list of diets in the accompanying table is not intended to be exhaustive.[Figure caption and citation for the preceding image starts]: Dietary options, part 1 of 2Courtesy of Mark Carlson, MD; used with permission [Citation ends].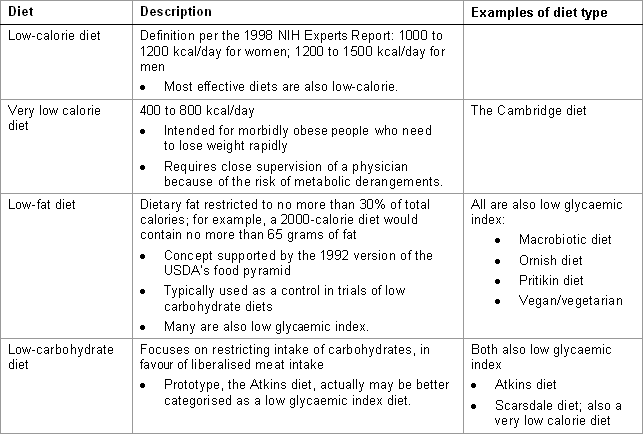 [Figure caption and citation for the preceding image starts]: Dietary options, part 2 of 2Courtesy of Mark Carlson, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Dietary options, part 2 of 2Courtesy of Mark Carlson, MD; used with permission [Citation ends].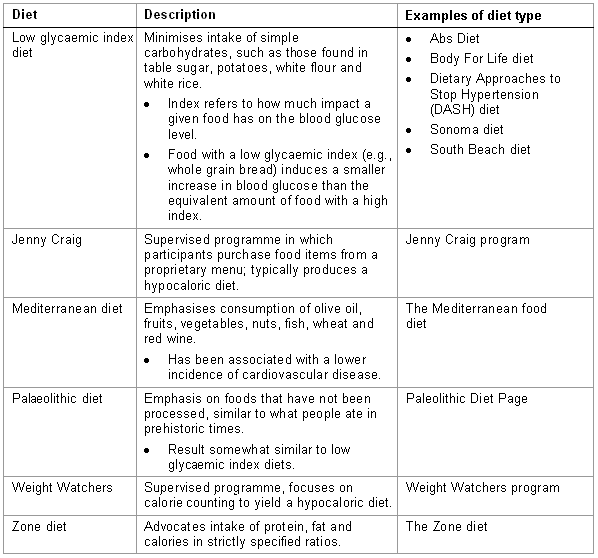
Increase in physical activity
Meta-analyses have indicated that weight loss is greater in diet plus exercise regimens than in diet-only regimens.[102]Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755-67.
http://www.ncbi.nlm.nih.gov/pubmed/17904936?tool=bestpractice.com
[112]Wu T, Gao X, Chen M, et al. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009 May;10(3):313-23.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1467-789X.2008.00547.x
http://www.ncbi.nlm.nih.gov/pubmed/19175510?tool=bestpractice.com
Exercise regimens alone without reduced-calorie diets are not effective for weight loss.[102]Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755-67.
http://www.ncbi.nlm.nih.gov/pubmed/17904936?tool=bestpractice.com
If the patient is physically capable, moderate physical exercise is introduced with 5 sessions per week for 30 minutes per session, in combination with strength training.[113]Semlitsch T, Stigler FL, Jeitler K, et al. Management of overweight and obesity in primary care - a systematic overview of international evidence-based guidelines. Obes Rev. 2019 Sep;20(9):1218-30.
https://onlinelibrary.wiley.com/doi/full/10.1111/obr.12889
http://www.ncbi.nlm.nih.gov/pubmed/31286668?tool=bestpractice.com
[114]Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018 Nov 20;320(19):2020-8.
http://www.ncbi.nlm.nih.gov/pubmed/30418471?tool=bestpractice.com
This can be increased as tolerated. In addition, an increase in other physical activity (e.g., taking the stairs instead of the lift) should be encouraged.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
[101]Shaw K, Gennat H, O'Rourke P, et al. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD003817.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003817.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/17054187?tool=bestpractice.com
An abundance of supervised and non-supervised exercise programs are available in which a patient could participate. World Health Organization guidelines for physical activity in adults of all age groups are outlined in the primary prevention section, and may be applicable to those seeking treatment for obesity.[92]Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020 Dec;54(24):1451-62.
https://bjsm.bmj.com/content/54/24/1451
http://www.ncbi.nlm.nih.gov/pubmed/33239350?tool=bestpractice.com
See Primary prevention.
A description of common physical activities and their respective rate of caloric expenditure is given, so that the healthcare provider can appreciate the level of expenditure that various physical activities can produce.
[Figure caption and citation for the preceding image starts]: Physical activitiesCourtesy of Mark Carlson, MD; used with permission [Citation ends].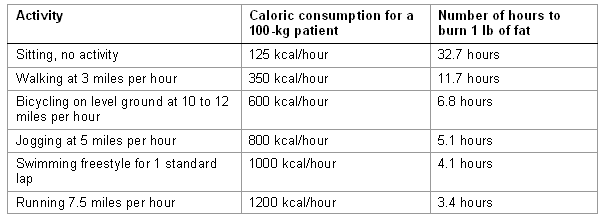
Adjunctive psychological therapy
Adding psychological therapy is an effective adjunct to diet and exercise, and is recommended in all receptive patients.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
[115]Booth HP, Prevost TA, Wright AJ, et al. Effectiveness of behavioural weight loss interventions delivered in a primary care setting: a systematic review and meta-analysis. Fam Pract. 2014 Dec;31(6):643-53.
https://academic.oup.com/fampra/article/31/6/643/592646
http://www.ncbi.nlm.nih.gov/pubmed/25298510?tool=bestpractice.com
[116]Södlerlund A, Fischer A, Johansson T. Physical activity, diet and behaviour modification in the treatment of overweight and obese adults: a systematic review. Perspect Public Health. 2009 May;129(3):132-42.
http://www.ncbi.nlm.nih.gov/pubmed/19514637?tool=bestpractice.com
Psychological intervention appears to be most effective when it is in the form of behavioural or cognitive behavioural therapy, and is prescribed as an adjunct to diet and exercise.[117]Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018 Sep 18;320(11):1163-71.
https://jamanetwork.com/journals/jama/fullarticle/2702878
http://www.ncbi.nlm.nih.gov/pubmed/30326502?tool=bestpractice.com
[118]Castelnuovo G, Pietrabissa G, Manzoni GM, et al. Cognitive behavioral therapy to aid weight loss in obese patients: current perspectives. Psychol Res Behav Manag. 2017;10:165-73.
https://www.dovepress.com/cognitive-behavioral-therapy-to-aid-weight-loss-in-obese-patients-curr-peer-reviewed-fulltext-article-PRBM
http://www.ncbi.nlm.nih.gov/pubmed/28652832?tool=bestpractice.com
Psychological therapy also seems to be best when given in person by a therapist compared with self-directed therapy.[119]Svetkey LP, Stevens VJ, Brantley PJ, et al; Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008 Mar 12;299(10):1139-48.
https://jamanetwork.com/journals/jama/fullarticle/181605
http://www.ncbi.nlm.nih.gov/pubmed/18334689?tool=bestpractice.com
In addition, the practice of frequent self-weighing appears to have a beneficial effect on weight loss.[120]Vanwormer JJ, French SA, Pereira MA, et al. The impact of regular self-weighing on weight management: a systematic literature review. Int J Behav Nutr Phys Act. 2008 Nov 4;5:54.
https://ijbnpa.biomedcentral.com/articles/10.1186/1479-5868-5-54
http://www.ncbi.nlm.nih.gov/pubmed/18983667?tool=bestpractice.com
Web-based behavioural interventions may provide useful educational tools for the achievement and maintenance of weight loss, and the prevention of excessive weight gain.[121]Manzoni GM, Pagnini F, Corti S, et al. Internet-based behavioral interventions for obesity: an updated systematic review. Clin Pract Epidemiol Ment Health. 2011 Mar 4;7:19-28.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3087973
http://www.ncbi.nlm.nih.gov/pubmed/21552423?tool=bestpractice.com
[122]Maon S, Edirippulige S, Ware R, et al. The use of web-based interventions to prevent excessive weight gain. J Telemed Telecare. 2012 Jan;18(1):37-41.
http://www.ncbi.nlm.nih.gov/pubmed/22101608?tool=bestpractice.com
[123]Crane MM, Lutes LD, Ward DS, et al. A randomized trial testing the efficacy of a novel approach to weight loss among men with overweight and obesity. Obesity (Silver Spring). 2015 Dec;23(12):2398-405.
https://onlinelibrary.wiley.com/doi/full/10.1002/oby.21265
http://www.ncbi.nlm.nih.gov/pubmed/26727117?tool=bestpractice.com
Access to social media has led to the adoption of these web-based applications for exercise and dietary coaching for weight loss and management; however, not all apps are created equal, and overall, evidence into their efficacy for individual sustained weight loss is lacking.[124]Chen J, Cade JE, Allman-Farinelli M. The most popular smartphone apps for weight loss: a quality assessment. JMIR Mhealth Uhealth. 2015 Dec 16;3(4):e104.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4704947
http://www.ncbi.nlm.nih.gov/pubmed/26678569?tool=bestpractice.com
In one review of 28 top-rated weight-loss applications, Noom was given the highest total score based on five independent ranking categories.[124]Chen J, Cade JE, Allman-Farinelli M. The most popular smartphone apps for weight loss: a quality assessment. JMIR Mhealth Uhealth. 2015 Dec 16;3(4):e104.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4704947
http://www.ncbi.nlm.nih.gov/pubmed/26678569?tool=bestpractice.com
However, even with Noom, the overall efficacy of total and sustained weight loss was most correlated with frequent and sustained engagement by each individual user.[125]Carey A, Yang Q, DeLuca L, et al. The relationship between weight loss outcomes and engagement in a mobile behavioral change intervention: retrospective analysis. JMIR Mhealth Uhealth. 2021 Nov 8;9(11):e30622.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8663454
http://www.ncbi.nlm.nih.gov/pubmed/34747706?tool=bestpractice.com
In addition, interactive social media platforms that bring together a community to achieve a specific health goal have been found to increase physical activity, weight loss, and overall wellbeing scores. These effects may be amplified when online platforms are combined with regular follow-up on progress from another support provider, either via telephone or in person.[126]Petkovic J, Duench S, Trawin J, et al. Behavioural interventions delivered through interactive social media for health behaviour change, health outcomes, and health equity in the adult population. Cochrane Database Syst Rev. 2021 May 31;(5):CD012932.
https://www.doi.org/10.1002/14651858.CD012932.pub2
http://www.ncbi.nlm.nih.gov/pubmed/34057201?tool=bestpractice.com
[127]Baer HJ, Rozenblum R, De La Cruz BA, et al. Effect of an online weight management program integrated with population health management on weight change: a randomized clinical trial. JAMA. 2020 Nov 3;324(17):1737-46.
https://www.doi.org/10.1001/jama.2020.18977
http://www.ncbi.nlm.nih.gov/pubmed/33141209?tool=bestpractice.com
Despite their initial promise, however, further research is needed to determine the long-term effectiveness of such interventions.
Adjunctive pharmacotherapy
Pharmacotherapy is recommended as an adjunct to diet and exercise in people whose BMI is ≥30 kg/m², or >27 kg/m² if associated with obesity-related comorbidity.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
[98]Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016 Jul;22 Suppl 3:1-203.
https://www.endocrinepractice.org/article/S1530-891X(20)44630-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27219496?tool=bestpractice.com
[128]Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022 Nov;163(5):1198-1225.
https://www.gastrojournal.org/article/S0016-5085(22)01026-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36273831?tool=bestpractice.com
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist targeting areas of the brain that regulate appetite and food intake.[129]Bergmann NC, Davies MJ, Lingvay I, et al. Semaglutide for the treatment of overweight and obesity: a review. Diabetes Obes Metab. 2023 Jan;25(1):18-35.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10092086
http://www.ncbi.nlm.nih.gov/pubmed/36254579?tool=bestpractice.com
Originally approved for the treatment of type 2 diabetes, semaglutide is now also indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial BMI ≥30 kg/m², or ≥27 kg/m² in the presence of at least one weight-related comorbidity.[130]Yanovski SZ, Yanovski JA. Progress in pharmacotherapy for obesity. JAMA. 2021 Jul 13;326(2):129-30.
http://www.ncbi.nlm.nih.gov/pubmed/34160571?tool=bestpractice.com
In the UK, the National Institute for Health and Care Excellence (NICE) recommends the use of semaglutide in adults as an adjunct to lifestyle measures only when it is used:
for a maximum of 2 years
within a specialist weight management service
in patients who have at least 1 weight-related comorbidity and have BMI ≥35 kg/m² (or a BMI between 30 kg/m² to <35 kg/m² who meet the criteria for referral to specialist weight management services).[131]National Institute for Health and Care Excellence. Semaglutide for managing overweight and obesity [TA875]. March 2023 [internet publication].
https://www.nice.org.uk/guidance/ta875
NICE recommends using lower BMI thresholds (usually reduced by 2.5 kg/m²) for people from South Asian, Chinese, other Asian, Middle Eastern, Black African or African-Caribbean family backgrounds.
Semaglutide is administered as a weekly subcutaneous injection. Randomised controlled trial (RCT) data showed that patients receiving semaglutide lost an average of 6% to 16% of their total body weight compared with controls, when combined with other behavioural modifications.[132]Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021 Apr 13;325(14):1414-25.
https://www.doi.org/10.1001/jama.2021.3224
http://www.ncbi.nlm.nih.gov/pubmed/33755728?tool=bestpractice.com
[133]Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021 Apr 13;325(14):1403-13.
https://www.doi.org/10.1001/jama.2021.1831
http://www.ncbi.nlm.nih.gov/pubmed/33625476?tool=bestpractice.com
Semaglutide has also demonstrated cardiovascular benefits; RCT data showed that in adults aged 45 and older with overweight or obesity who have concurrent cardiovascular disease (but no history of diabetes), semaglutide reduces the overall risk of major cardiac events (heart attack, stroke, or cardiovascular death) by 20% at a mean follow-up of 40 months.[134]Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023 Dec 14;389(24):2221-32.
http://www.ncbi.nlm.nih.gov/pubmed/37952131?tool=bestpractice.com
Based on these results, the Food and Drug Administration (FDA) has expanded its indication, granting approval for the use of semaglutide to reduce the risk of major cardiac events in adults with cardiovascular disease and either obesity or overweight. Common adverse effects include gastrointestinal disturbance, headache, fatigue, and hypoglycaemia in diabetic patients. Some evidence suggests that overall weight loss with semaglutide may include both a reduction in adiposity as well as a reduction in fat-free mass (a surrogate marker for muscle mass); however, the long-term implications of this are currently unclear.[135]Ida S, Kaneko R, Imataka K, et al. Effects of antidiabetic drugs on muscle mass in type 2 diabetes mellitus. Curr Diabetes Rev. 2021;17(3):293-303.
http://www.ncbi.nlm.nih.gov/pubmed/32628589?tool=bestpractice.com
Semaglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma and patients with multiple endocrine neoplasia syndrome type 2 due to an increased risk of medullary thyroid cancer. Semaglutide is also available as an oral formulation; however, this is only currently approved for the management of type 2 diabetes. See Emerging treatments.
Liraglutide, another GLP-1 receptor agonist, is approved for the same indication as semaglutide. In the UK, NICE recommends liraglutide as an option for adults only if they have a BMI ≥35 kg/m² (or ≥32.5 kg/m² for members of ethnic groups known to be at greater risk), non-diabetic hyperglycaemia, a high risk of cardiovascular disease, and if prescribed by a specialist weight management service.[136]National Institute for Health and Care Excellence. Liraglutide for managing overweight and obesity [TA664]. December 2020 [internet publication].
https://www.nice.org.uk/guidance/ta664
Liraglutide is also administered as a subcutaneous injection, but requires daily administration. When combined with lifestyle interventions, such as participating in a regular physical activity programme, liraglutide has demonstrated efficacy in reducing weight and improving metabolic control in a number of clinical trials involving obese or overweight patients with or without diabetes.[137]Isaacs D, Prasad-Reddy L, Srivastava SB. Role of glucagon-like peptide 1 receptor agonists in management of obesity. Am J Health Syst Pharm. 2016 Oct 1;73(19):1493-507.
http://www.ncbi.nlm.nih.gov/pubmed/27521241?tool=bestpractice.com
[138]Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015 Aug 18;314(7):687-99.
http://www.ncbi.nlm.nih.gov/pubmed/26284720?tool=bestpractice.com
[139]Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015 Jul 2;373(1):11-22.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411892
http://www.ncbi.nlm.nih.gov/pubmed/26132939?tool=bestpractice.com
Weight loss is significantly increased when liraglutide is combined with exercise compared with lifestyle interventions or liraglutide therapy alone.[140]Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021 May 6;384(18):1719-30.
https://www.doi.org/10.1056/NEJMoa2028198
http://www.ncbi.nlm.nih.gov/pubmed/33951361?tool=bestpractice.com
RCTs of liraglutide have been conducted as part of the Satiety and Clinical Adiposity-Liraglutide Evidence in Nondiabetic and Diabetic Individuals (SCALE) programme.[141]Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond). 2013 Nov;37(11):1443-51.
http://www.ncbi.nlm.nih.gov/pubmed/23812094?tool=bestpractice.com
The contraindications and warnings and safety profile of liraglutide is similar to that of semaglutide.[130]Yanovski SZ, Yanovski JA. Progress in pharmacotherapy for obesity. JAMA. 2021 Jul 13;326(2):129-30.
http://www.ncbi.nlm.nih.gov/pubmed/34160571?tool=bestpractice.com
However, in one trial comparing use of semaglutide or liraglutide in addition to behavioural modifications, patients receiving semaglutide had significantly greater weight loss.[142]Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022 Jan 11;327(2):138-50.
http://www.ncbi.nlm.nih.gov/pubmed/35015037?tool=bestpractice.com
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist that is also administered as a weekly subcutaneous injection. It is approved for the same indications as semaglutide. RCT evidence suggests that, when combined with intensive lifestyle modifications, patients receiving a weekly dose of tirzepatide achieved weight loss of around 18% of their total body weight.[143]Wadden TA, Chao AM, Machineni S, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023 Nov;29(11):2909-18.
https://www.nature.com/articles/s41591-023-02597-w
http://www.ncbi.nlm.nih.gov/pubmed/37840095?tool=bestpractice.com
[144]Abbasi J. FDA Green-Lights Tirzepatide, Marketed as Zepbound, for chronic weight management. JAMA. 2023 Dec 12;330(22):2143-4.
https://jamanetwork.com/journals/jama/article-abstract/2812190
http://www.ncbi.nlm.nih.gov/pubmed/37966831?tool=bestpractice.com
In another RCT, patients who received tirzepatide experienced an average weight reduction of 20.9%.[145]Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022 Jul 21;387(3):205-16.
https://www.nejm.org/doi/10.1056/NEJMoa2206038?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/35658024?tool=bestpractice.com
A dose-dependent reduction in weight was demonstrated in both studies.[143]Wadden TA, Chao AM, Machineni S, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023 Nov;29(11):2909-18.
https://www.nature.com/articles/s41591-023-02597-w
http://www.ncbi.nlm.nih.gov/pubmed/37840095?tool=bestpractice.com
[145]Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022 Jul 21;387(3):205-16.
https://www.nejm.org/doi/10.1056/NEJMoa2206038?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/35658024?tool=bestpractice.com
Tirzepatide has the same contraindications and warnings, and a similar adverse effect profile to other GLP-1 receptor agonists.[146]Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021 Jul 10;398(10295):143-55.
http://www.ncbi.nlm.nih.gov/pubmed/34186022?tool=bestpractice.com
Note that tirzepatide should not be used concurrently with a GLP-1 receptor agonist.
Orlistat is an oral inhibitor of fat absorption (inhibitor of gastric and pancreatic lipase). It is approved for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity. It has been shown to have modest effectiveness (about 5% loss in body weight) when combined with diet and exercise alone, but mild gastrointestinal side effects (including diarrhoea) are common.[147]Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016 Jun 14;315(22):2424-34.
https://jamanetwork.com/journals/jama/fullarticle/2528211
http://www.ncbi.nlm.nih.gov/pubmed/27299618?tool=bestpractice.com
American Gastroenterological Association (AGA) guidelines recommend against the use of orlistat, but note that it may be reasonable if the patient values modest weight loss over possible gastrointestinal adverse events.[128]Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022 Nov;163(5):1198-1225.
https://www.gastrojournal.org/article/S0016-5085(22)01026-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36273831?tool=bestpractice.com
Combining orlistat with L-carnitine may offer better results than orlistat as a monotherapy.[148]Derosa G, Maffioli P, Ferrari I, et al. Orlistat and L-carnitine compared to orlistat alone on insulin resistance in obese diabetic patients. Endocr J. 2010;57(9):777-86.
https://www.jstage.jst.go.jp/article/endocrj/57/9/57_K10E-049/_pdf
http://www.ncbi.nlm.nih.gov/pubmed/20683173?tool=bestpractice.com
Patients who use orlistat should take a multivitamin containing fat-soluble vitamins at least 2 hours before or after orlistat.[128]Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022 Nov;163(5):1198-1225.
https://www.gastrojournal.org/article/S0016-5085(22)01026-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36273831?tool=bestpractice.com
Naltrexone/bupropion is an oral combination therapy containing naltrexone, an opioid receptor antagonist, plus bupropion, a noradrenaline-dopamine reuptake inhibitor. It is approved for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity. In clinical trials, naltrexone/bupropion was effective in reducing weight compared with placebo when administered in conjunction with lifestyle interventions.[149]Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010 Aug 21;376(9741):595-605.
http://www.ncbi.nlm.nih.gov/pubmed/20673995?tool=bestpractice.com
[150]Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009 Dec;94(12):4898-906.
http://www.ncbi.nlm.nih.gov/pubmed/19846734?tool=bestpractice.com
[151]Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013 May;21(5):935-43.
https://onlinelibrary.wiley.com/doi/full/10.1002/oby.20309
http://www.ncbi.nlm.nih.gov/pubmed/23408728?tool=bestpractice.com
[152]Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011 Jan;19(1):110-20.
https://onlinelibrary.wiley.com/doi/full/10.1038/oby.2010.147
http://www.ncbi.nlm.nih.gov/pubmed/20559296?tool=bestpractice.com
[153]Hollander P, Gupta AK, Plodkowski R, et al; COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013 Dec;36(12):4022-9.
https://care.diabetesjournals.org/content/36/12/4022.long
http://www.ncbi.nlm.nih.gov/pubmed/24144653?tool=bestpractice.com
Naltrexone/bupropion should be avoided in patients with seizure disorders or substance misuse disorders. It should be used with caution and at lower doses in patients with hepatic or renal impairment.
Phentermine/topiramate is an oral combination therapy containing phentermine, an amfetamine/appetite suppressant, and topiramate, an anticonvulsant. It is approved for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity. Phentermine/topiramate is effective in reducing weight when combined with lifestyle interventions, and may also improve markers of cardiovascular system function such as blood pressure, total cholesterol, triglycerides, and blood glucose levels.[154]Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011 Apr 16;377(9774):1341-52.
http://www.ncbi.nlm.nih.gov/pubmed/21481449?tool=bestpractice.com
[155]Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012 Feb;20(2):330-42.
https://onlinelibrary.wiley.com/doi/full/10.1038/oby.2011.330
http://www.ncbi.nlm.nih.gov/pubmed/22051941?tool=bestpractice.com
[156]Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012 Feb;95(2):297-308.
https://academic.oup.com/ajcn/article/95/2/297/4576717
http://www.ncbi.nlm.nih.gov/pubmed/22158731?tool=bestpractice.com
[157]Lei XG, Ruan JQ, Lai C, et al. Efficacy and safety of phentermine/topiramate in adults with overweight or obesity: a systematic review and meta-analysis. Obesity (Silver Spring). 2021 Jun;29(6):985-94.
http://www.ncbi.nlm.nih.gov/pubmed/33864346?tool=bestpractice.com
Phentermine/topiramate should be used with caution and at lower doses in patients with hepatic or renal impairment, and in those with hypertension or arrhythmias, due to the adrenergic effects of phentermine. Topiramate is associated with congenital malformations; women of childbearing potential should be counselled on effective contraception.[128]Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022 Nov;163(5):1198-1225.
https://www.gastrojournal.org/article/S0016-5085(22)01026-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36273831?tool=bestpractice.com
Phentermine is a controlled substance due to its abuse potential, and should not be prescribed for patients with a history of substance misuse.
Setmelanotide is a melanocortin 4 (MC4) receptor agonist approved for certain rare genetic conditions that can cause obesity at an early age.[158]Markham A. Setmelanotide: first approval. Drugs. 2021 Feb;81(3):397-403.
http://www.ncbi.nlm.nih.gov/pubmed/33638809?tool=bestpractice.com
Patients with confirmed genetic testing for pro-opiomelanocortin (POMC), proprotein subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency are candidates for setmelanotide.[158]Markham A. Setmelanotide: first approval. Drugs. 2021 Feb;81(3):397-403.
http://www.ncbi.nlm.nih.gov/pubmed/33638809?tool=bestpractice.com
[159]National Institute for Health and Care Excellence. Setmelanotide for treating obesity caused by LEPR or POMC deficiency [HST21]. July 2022 [internet publication].
https://www.nice.org.uk/guidance/hst21
Setmelanotide works by activating the MC4 receptor, which then initiates a signalling cascade that promotes a feeling of fullness after eating, therefore decreasing excessive caloric intake.[160]Clément K, van den Akker E, Argente J, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020 Dec;8(12):960-70.
http://www.ncbi.nlm.nih.gov/pubmed/33137293?tool=bestpractice.com
Common side effects include gastrointestinal disturbances, headache, injection-site reactions, and skin hyperpigmentation.[158]Markham A. Setmelanotide: first approval. Drugs. 2021 Feb;81(3):397-403.
http://www.ncbi.nlm.nih.gov/pubmed/33638809?tool=bestpractice.com
Setmelanotide is also approved for the treatment of obesity and hunger control in patients with confirmed Bardet-Biedl syndrome.[161]Haqq AM, Chung WK, Dollfus H, et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alström syndrome: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 2022 Dec;10(12):859-68.
http://www.ncbi.nlm.nih.gov/pubmed/36356613?tool=bestpractice.com
The choice of pharmacotherapy for the treatment of obesity is dependent on multiple variables. Patient comorbidities such as hepatic or renal impairment, history of substance misuse, seizure disorders, and some genetic conditions may prohibit use of one or more of the discussed medications. Moreover, injectable medications may not be appropriate for patients who are unable to administer them safely and independently.
Semaglutide and liraglutide are first-line pharmacotherapy options with clinically proven weight loss and cardiometabolic effects, provided there are no contraindications.[128]Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022 Nov;163(5):1198-1225.
https://www.gastrojournal.org/article/S0016-5085(22)01026-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36273831?tool=bestpractice.com
They are both approved for long-term use. Setmelanotide is a first-line option for patients with POMC, PCSK1, LEPR deficiency, or Bardet-Biedl syndrome.
If semaglutide or liraglutide are contraindicated or not tolerated, second-line options include naltrexone/bupropion and phentermine/topiramate. These options may also be beneficial in patients with certain comorbidities. For example, in patients with obesity who also suffer migraine headaches, phentermine/topiramate may be considered as topiramate also treats migraines. Naltrexone/bupropion may be considered in patients with obesity who also desire pharmacological aid in smoking cessation, or in patients with depression.[128]Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022 Nov;163(5):1198-1225.
https://www.gastrojournal.org/article/S0016-5085(22)01026-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36273831?tool=bestpractice.com
All of these agents are approved for long-term use. If first- or second-line options are not tolerated, a third-line option is orlistat if the patient values modest weight loss over the possible gastrointestinal side effects of this drug.[128]Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022 Nov;163(5):1198-1225.
https://www.gastrojournal.org/article/S0016-5085(22)01026-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36273831?tool=bestpractice.com
Phentermine monotherapy is approved for short-term use only, and is therefore a third-line treatment option.
Lorcaserin (a serotonin receptor type agonist) had been used for the treatment of obesity in adults, but was withdrawn from the US market because of an increased risk of cancer.[162]Food and Drug Adminstration. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. February 2020 [internet publication].
https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
Lorcaserin was discontinued before attaining approval in Europe.
Bariatric surgery
In general, bariatric surgery (i.e., weight reduction surgery) reduces caloric intake by altering hunger and fullness through surgical alteration of the stomach, small intestine, or both, which produces weight loss. However, the precise mechanism(s) through which a given bariatric procedure results in weight loss is incompletely understood and is undergoing active investigation.[163]Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020 Sep 1;324(9):879-87.
http://www.ncbi.nlm.nih.gov/pubmed/32870301?tool=bestpractice.com
[164]Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Metabolic and endocrine consequences of bariatric surgery. Front Endocrinol (Lausanne). 2019 Sep 19;10:626.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6761298
http://www.ncbi.nlm.nih.gov/pubmed/31608009?tool=bestpractice.com
[165]Osorio-Conles Ó, Vidal J, de Hollanda A. Impact of bariatric surgery on adipose tissue biology. J Clin Med. 2021 Nov 25;10(23):5516.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8658722
http://www.ncbi.nlm.nih.gov/pubmed/34884217?tool=bestpractice.com
One large meta-analysis of outcomes in metabolic-bariatric surgery found that obese patients who underwent bariatric surgery had significantly lower rates of all-cause mortality, compared with obese non-operated controls.[166]Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021 May 15;397(10287):1830-41.
http://www.ncbi.nlm.nih.gov/pubmed/33965067?tool=bestpractice.com
This effect was more pronounced in patients with preoperative diabetes than in those without diabetes.[166]Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021 May 15;397(10287):1830-41.
http://www.ncbi.nlm.nih.gov/pubmed/33965067?tool=bestpractice.com
Evidence is accumulating that bariatric surgery is an effective treatment for type 2 diabetes and hypertension in obese patients.[167]Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014 Aug 8;(8):CD003641.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003641.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/25105982?tool=bestpractice.com
[168]Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009 Mar;122(3):248-56.
http://www.ncbi.nlm.nih.gov/pubmed/19272486?tool=bestpractice.com
[169]Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009 Sep 28;169(17):1619-26.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879275
http://www.ncbi.nlm.nih.gov/pubmed/19786682?tool=bestpractice.com
The risk of death following bariatric procedures is approximately 0.1%.[170]American Society for Metabolic and Bariatric Surgery. Metabolic and bariatric surgery fact sheet. July 2021 [internet publication].
https://asmbs.org/resources/metabolic-and-bariatric-surgery
The overall rate of major complication is approximately 4%.[170]American Society for Metabolic and Bariatric Surgery. Metabolic and bariatric surgery fact sheet. July 2021 [internet publication].
https://asmbs.org/resources/metabolic-and-bariatric-surgery
According to a National Institutes of Health consensus statement from 1991, patients with a BMI ≥40 kg/m² (i.e., class III obesity), or ≥35 kg/m² with obesity-related comorbidity (e.g., hypertension, diabetes, sleep apnoea, GORD) may be candidates for most bariatric procedures.[1]Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 suppl 2):S102-38.
https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437739.71477.ee
http://www.ncbi.nlm.nih.gov/pubmed/24222017?tool=bestpractice.com
[167]Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014 Aug 8;(8):CD003641.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003641.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/25105982?tool=bestpractice.com
[171]Buchwald H. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005 May-Jun;1(3):371-81.
http://www.ncbi.nlm.nih.gov/pubmed/16925250?tool=bestpractice.com
[172]Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005 Apr 5;142(7):547-59.
https://annals.org/aim/fullarticle/718311/meta-analysis-surgical-treatment-obesity
http://www.ncbi.nlm.nih.gov/pubmed/15809466?tool=bestpractice.com
However, these guidelines are now over 30 years old. The increased use of minimally invasive (i.e., laparoscopic) approaches has decreased both the morbidity and mortality associated with these operations. This has contributed to an overall broadening of bariatric indications. As of 2022, the American Society for Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders recommend bariatric surgery for patients with:[173]Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) indications for metabolic and bariatric surgery. Obes Surg. 2023 Jan;33(1):3-14.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9834364
http://www.ncbi.nlm.nih.gov/pubmed/36336720?tool=bestpractice.com
BMI ≥35 kg/m² with or without comorbidities
BMI ≥30 kg/m² and type 2 diabetes mellitus
BMI of 30-34.9 kg/m² (class I obesity) who do not achieve substantial durable weight loss or comorbidity improvement with non-surgical management.
BMI thresholds do not apply equally to all populations, so bariatric surgery may also be considered for some individuals with lower BMI (e.g., in Asian populations, clinical obesity is defined as BMI >25 kg/m²).[173]Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) indications for metabolic and bariatric surgery. Obes Surg. 2023 Jan;33(1):3-14.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9834364
http://www.ncbi.nlm.nih.gov/pubmed/36336720?tool=bestpractice.com
There is no upper age limit for bariatric surgery, but patients should be carefully assessed for comorbidities and frailty.[173]Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) indications for metabolic and bariatric surgery. Obes Surg. 2023 Jan;33(1):3-14.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9834364
http://www.ncbi.nlm.nih.gov/pubmed/36336720?tool=bestpractice.com
Loss of up to 70% of excess body weight has been observed in the first 2 years following bariatric surgery, with significant improvement and even total remission in weight-related comorbidities such as diabetes, hypertension, obstructive sleep apnoea, dyslipidaemia, and cardiovascular disease.[170]American Society for Metabolic and Bariatric Surgery. Metabolic and bariatric surgery fact sheet. July 2021 [internet publication].
https://asmbs.org/resources/metabolic-and-bariatric-surgery
In addition, bariatric operations are as safe as many other commonly performed laparoscopic procedures today.[170]American Society for Metabolic and Bariatric Surgery. Metabolic and bariatric surgery fact sheet. July 2021 [internet publication].
https://asmbs.org/resources/metabolic-and-bariatric-surgery
[174]Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017 Feb 16;376(7):641-51.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5451258
http://www.ncbi.nlm.nih.gov/pubmed/28199805?tool=bestpractice.com
[175]Berry MA, Urrutia L, Lamoza P, et al. Sleeve gastrectomy outcomes in patients with BMI between 30 and 35 - 3 years of follow-up. Obes Surg. 2018 Mar;28(3):649-55.
https://www.doi.org/10.1007/s11695-017-2897-x
http://www.ncbi.nlm.nih.gov/pubmed/28975492?tool=bestpractice.com
One systematic review found that bariatric surgery is also associated with reduced postoperative prevalence and severity of depression.[176]Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016 Jan 12;315(2):150-63.
https://jamanetwork.com/journals/jama/fullarticle/2481004
http://www.ncbi.nlm.nih.gov/pubmed/26757464?tool=bestpractice.com
In 2019, sleeve gastrectomy (59.4%) was the most commonly performed bariatric operation in the US, followed by gastric bypass (17.8%), revisional procedures (16.7%), intragastric balloons (2.4%), adjustable gastric banding (0.9%), and biliopancreatic diversion/duodenal switch procedures (0.9%).[170]American Society for Metabolic and Bariatric Surgery. Metabolic and bariatric surgery fact sheet. July 2021 [internet publication].
https://asmbs.org/resources/metabolic-and-bariatric-surgery
Roux-en-Y gastric bypass may be more efficacious than gastric banding, but the latter may have less morbidity.[177]Tice JA, Karliner L, Walsh J, et al. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008 Oct;121(10):885-93.
http://www.ncbi.nlm.nih.gov/pubmed/18823860?tool=bestpractice.com
[178]Garb J, Welch G, Zagarins S, et al. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009 Oct;19(10):1447-55.
http://www.ncbi.nlm.nih.gov/pubmed/19655209?tool=bestpractice.com
[179]Nguyen NT, Slone JA, Nguyen XM, et al. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009 Oct;250(4):631-41.
http://www.ncbi.nlm.nih.gov/pubmed/19730234?tool=bestpractice.com
In one study assessing 5-year outcomes following Roux-en-Y gastric bypass or laparoscopic duodenal switch in patients with a BMI of 50 to 60 kg/m², duodenal switch resulted in greater weight loss compared with gastric bypass but was associated with more surgical, nutritional, and gastrointestinal adverse effects.[180]Risstad H, Søvik TT, Engström M, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg. 2015 Apr;150(4):352-61.
http://www.ncbi.nlm.nih.gov/pubmed/25650964?tool=bestpractice.com
Further trials are needed.
Laparoscopic sleeve gastrectomy has become the most commonly performed surgical treatment for obesity, primarily due to good short-term results. Sleeve gastrectomy produces more weight loss than adjustable gastric banding, but less than the gastric bypass.[181]ASMBS Clinical Issues Committee. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2012 May-Jun;8(3):e21-6.
http://www.ncbi.nlm.nih.gov/pubmed/22417852?tool=bestpractice.com
One study found that at 8 years postoperatively, sleeve gastrectomy patients had satisfying weight loss results, demonstrating a 55% mean reduction in excess body weight. However, almost 50% of patients had some degree of weight regain.[182]Ben-Porat T, Mashin L, Kaluti D, et al. Weight loss outcomes and lifestyle patterns following sleeve gastrectomy: an 8-year retrospective sStudy of 212 patients. Obes Surg. 2021 Nov;31(11):4836-45.
http://www.ncbi.nlm.nih.gov/pubmed/34403078?tool=bestpractice.com
Weight regain was positively correlated with increased postoperative maladaptive eating patterns and lifestyle habits.[182]Ben-Porat T, Mashin L, Kaluti D, et al. Weight loss outcomes and lifestyle patterns following sleeve gastrectomy: an 8-year retrospective sStudy of 212 patients. Obes Surg. 2021 Nov;31(11):4836-45.
http://www.ncbi.nlm.nih.gov/pubmed/34403078?tool=bestpractice.com
Another long-term outcomes study found that over 10 years, all bariatric procedure types were associated with sustained weight loss.[183]O'Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes afteradjustable gastric banding. Obes Surg. 2019 Jan;29(1):3-14.
https://www.doi.org/10.1007/s11695-018-3525-0
http://www.ncbi.nlm.nih.gov/pubmed/30293134?tool=bestpractice.com
However, some series show sleeve gastrectomy failure rates as high as 51.4% and conversion rates to Roux-en-Y gastric bypass, due to weight regain or reflux disease, as high as 36%.[184]Sepúlveda M, Alamo M, Saba J, et al. Long-term weight loss in laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2017 Oct;13(10):1676-81.
http://www.ncbi.nlm.nih.gov/pubmed/28807556?tool=bestpractice.com
[185]Felsenreich DM, Langer FB, Kefurt R, et al. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016 Nov;12(9):1655-62.
http://www.ncbi.nlm.nih.gov/pubmed/27317599?tool=bestpractice.com
Limited preliminary data have suggested that the intragastric balloon, in conjunction with dieting, may have short-term efficacy in weight loss.[186]Fernandes M, Atallah AN, Soares BG, et al. Intragastric balloon for obesity. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD004931.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004931.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/17253531?tool=bestpractice.com
[187]Imaz I, Martínez-Cervell C, García-Alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity: a meta-analysis. Obes Surg. 2008 Jul;18(7):841-6.
http://www.ncbi.nlm.nih.gov/pubmed/18459025?tool=bestpractice.com
[188]Muniraj T, Day LW, Teigen LM, et al. AGA clinical practice guidelines on intragastric balloons in the management of obesity. Gastroenterology. 2021 Apr;160(5):1799-808.
https://www.doi.org/10.1053/j.gastro.2021.03.003
http://www.ncbi.nlm.nih.gov/pubmed/33832655?tool=bestpractice.com
Intragastric balloons were initially associated with several devastating adverse events, causing removal from the US market.[189]Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis. 2016 Feb;12(2):430-5.
http://www.ncbi.nlm.nih.gov/pubmed/26775045?tool=bestpractice.com
However, newer models with filling mediums that include water and air have since been approved by the FDA and are being studied.[188]Muniraj T, Day LW, Teigen LM, et al. AGA clinical practice guidelines on intragastric balloons in the management of obesity. Gastroenterology. 2021 Apr;160(5):1799-808.
https://www.doi.org/10.1053/j.gastro.2021.03.003
http://www.ncbi.nlm.nih.gov/pubmed/33832655?tool=bestpractice.com
 ]
A combination of a reduced-calorie diet and exercise is more efficacious than either alone.[102] Additional weight loss may be possible with some medicine regimens.[102] The initial goal of weight loss therapy (diet and exercise) is a 10% reduction in body weight over a 6-month period.[1] This equates to a loss of 0.23 to 0.454 kg per week in the patient with a BMI of ≤35 kg/m², and 0.454 to 0.91 kg per week in the patient with a BMI of >35 kg/m². After the initial 6-month period, the patient is re-assessed to determine the efficacy of the therapy, whether the patient needs to lose more weight, or whether a weight-maintenance programme may be established.
]
A combination of a reduced-calorie diet and exercise is more efficacious than either alone.[102] Additional weight loss may be possible with some medicine regimens.[102] The initial goal of weight loss therapy (diet and exercise) is a 10% reduction in body weight over a 6-month period.[1] This equates to a loss of 0.23 to 0.454 kg per week in the patient with a BMI of ≤35 kg/m², and 0.454 to 0.91 kg per week in the patient with a BMI of >35 kg/m². After the initial 6-month period, the patient is re-assessed to determine the efficacy of the therapy, whether the patient needs to lose more weight, or whether a weight-maintenance programme may be established. ]
]
 [Figure caption and citation for the preceding image starts]: Dietary options, part 2 of 2Courtesy of Mark Carlson, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Dietary options, part 2 of 2Courtesy of Mark Carlson, MD; used with permission [Citation ends].
